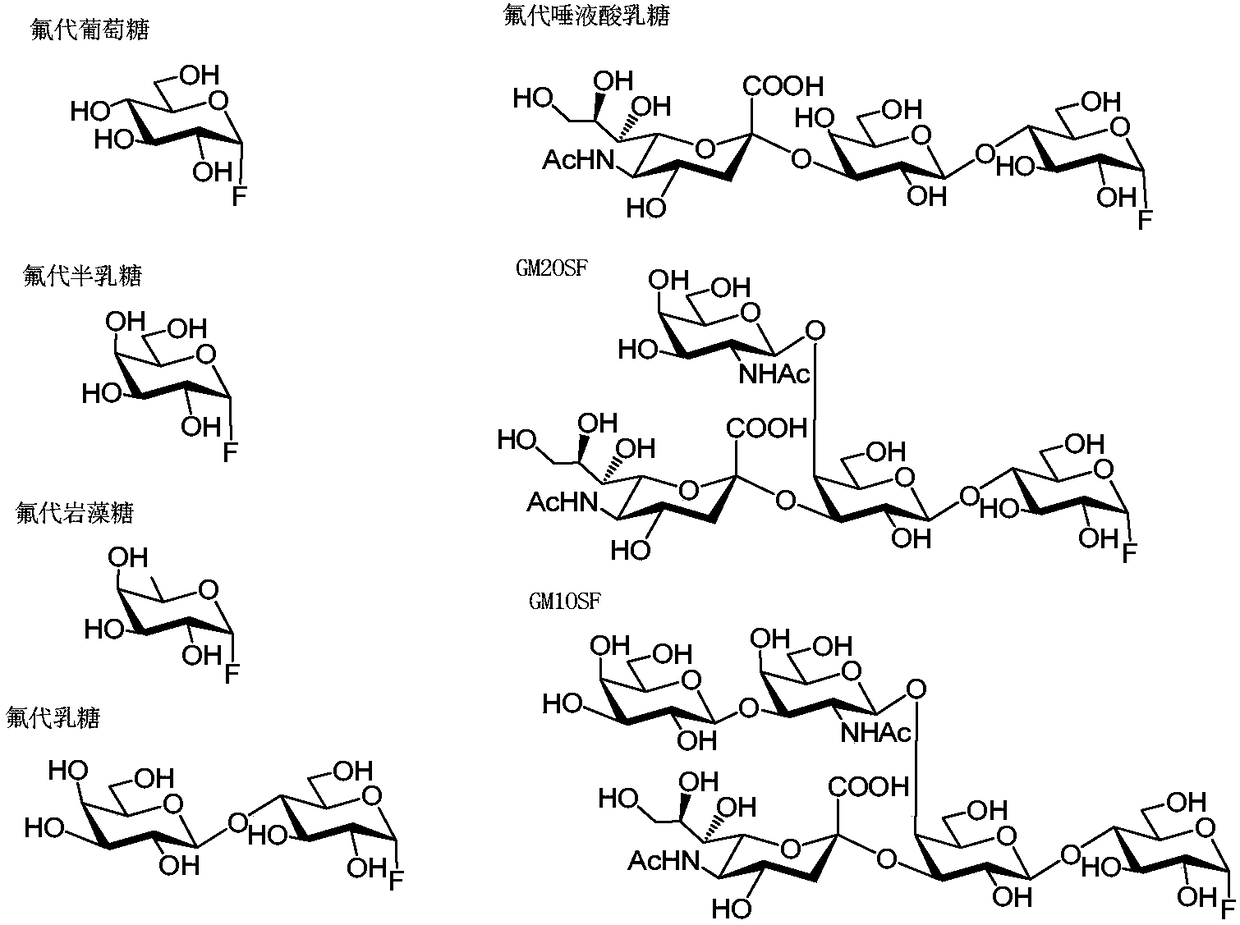
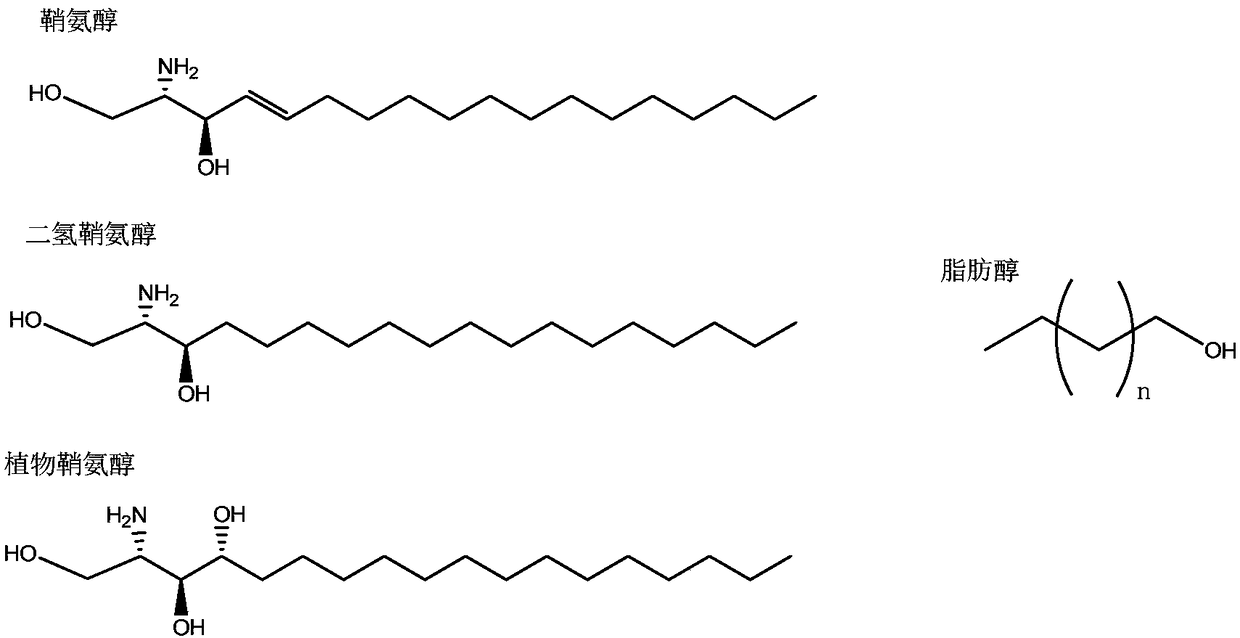
Novel glycosphingolipid endoglycosidase, and gene engineering preparation method and application thereof
An endoglycosidase and genetic engineering technology, which is applied in the application field of glycosphingolipid endoglycosidase and its genetic engineering preparation, analysis and synthesis of glycosphingolipids, can solve the problem of limited application range, narrow substrate spectrum, and activity low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1, the cloning of wild-type glycosphingolipid endoglycosidase 103S_EGCase I gene
[0070] Firstly, the polynucleotide sequence of 103S_EGCase I was cloned from the Rhodococcus equi 103S genome. Through codon optimization, it was unexpectedly found that the optimized sequence (SEQ ID NO: 1) can express a large amount of soluble glycosphingose in E. coli host Endolipid glycosidase I 103S_EGCase I (SEQ ID NO: 2) overcomes the disadvantage that the glycosphingolipid endoglycosidase EGCase I derived from Rhodococcus sp. M-750 cannot be solublely expressed, which limits its application. The specific cloning method is as follows:
[0071] Use upstream primer 5'-AAACGCGGATCCGCCCCGCCGGCGACCCCGATTAC-3'
[0072] (underlined base is restriction endonuclease BamH I recognition site, SEQ ID NO:3)
[0073] and downstream primer 5'-AAACCCAAGCTTTCAGGACGAACCGCTAC-3'
[0074] (the underlined base is the restriction endonuclease Hind III recognition site, SEQ ID NO: 4)
[...
Embodiment 2
[0076] Example 2 Expression, purification and activity determination of 103S_EGCase I
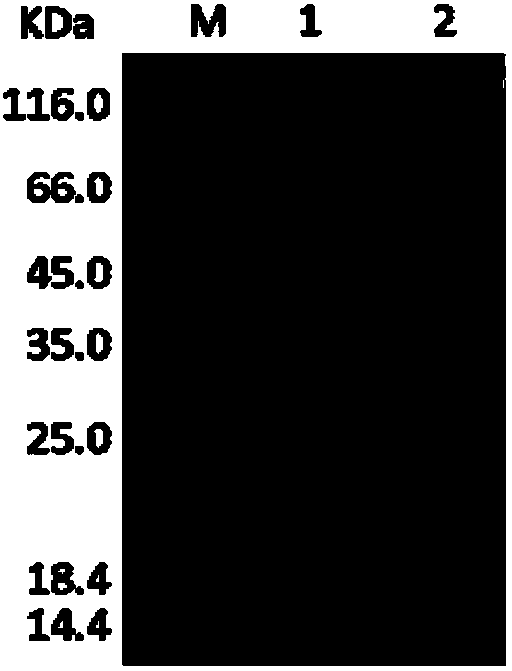
[0077] The engineered bacteria in the glycerol tube were inoculated into a 4 mL LB medium test tube containing 100 ug / mL kanamycin at a volume ratio of 1%, and cultured at 37° C. at 220 rpm for 12 hours. Transfer the 4mL bacterial liquid to a 1L LB medium shake flask containing 50ug / mL kanamycin, culture at 220rpm at 37°C for about 2.5h, make the OD600 reach about 0.9, add 0.1mM IPTG inducer, and induce at 25°C at 200rpm Cultivate for 12-16h. The Escherichia coli cell suspension harvested after fermentation was sonicated, and after one-step Ni-NTA affinity chromatography treatment, the target protein with a purity of more than 95% could be obtained ( image 3 ).
[0078] Using monosialotetrahexosyl ganglioside (GM1) as the substrate to test the hydrolysis activity of the recombinant enzyme, 10nmol substrate and an appropriate amount of enzyme solution in 20 μL of 50mM sodium acetate buffe...
Embodiment 3
[0079] Example 3 Design, Construction, Expression, Purification and Characterization of Synthetic Active Mutants of 103S_EGCase I
[0080] Site-directed mutation of the nucleophilic catalytic residue of glycoside hydrolase to an amino acid that does not have nucleophilic function, resulting in the loss of the original hydrolysis activity of the enzyme. Synthesis of glycosidic bonds, thus becoming glycoside synthase. Using sequence alignment, we determined that the nucleophilic catalytic residue of 103S_EGCase I was glutamic acid at position 339, and mutated it into a series of non-nucleophilic amino acids, including alanine, serine and methionine. Using the recombinant plasmid pET28a-103S_EGCase I as a template, and using a pair of complementary oligonucleotides with mutation sites as primers, use PrimeSTAR Mix high-fidelity enzyme (TakaRa Company) to perform PCR amplification of the whole plasmid to obtain specific mutation sites. Spot the recombinant plasmid. The primer se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com