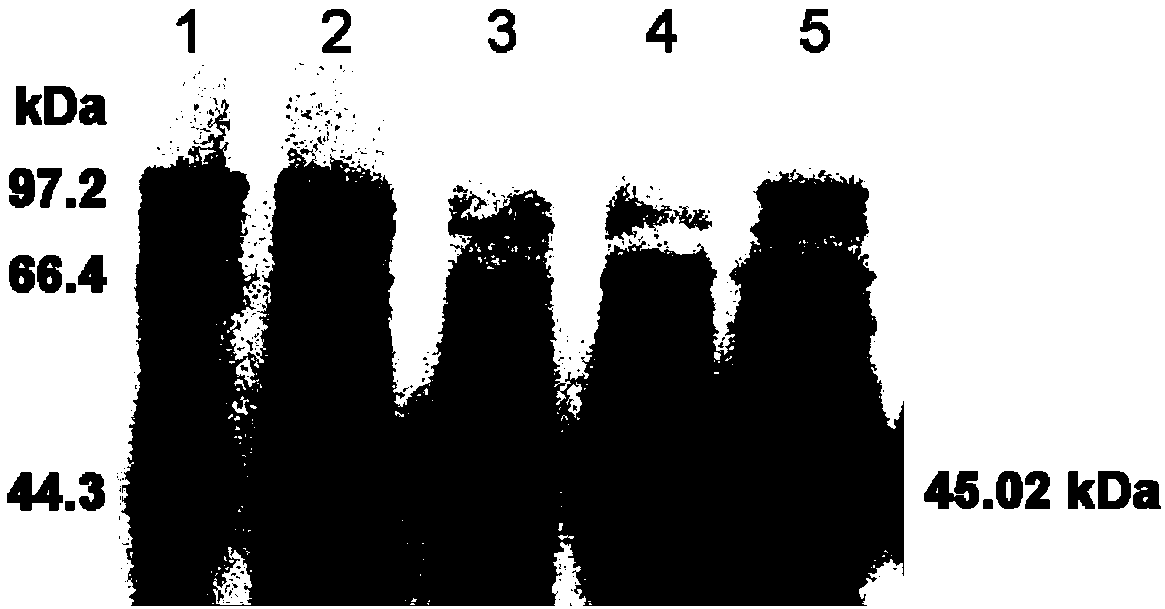Chalcone isomerase and coding gene, expression vector, host bacteria and application thereof
A technology of chalcone isomerase and coding gene, which is applied in the field of bioengineering, can solve problems such as limiting CHI regulation and improvement, limiting CHI evolution research, etc., and achieves good practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment provides a chalcone isomerase, the amino acid sequence of the chalcone isomerase is shown in SEQ ID NO.1, the estimated protein molecular weight is 25.018kD, and the isoelectric point is 4.95.
[0041] This embodiment also provides a gene encoding chalcone isomerase, and the base sequence of the gene encoding chalcone isomerase is shown in SEQ ID NO.2.
[0042] The coding gene of chalcone isomerase is obtained by amplifying the cDNA of the CHI gene that controls the synthesis of anthocyanins in the Japanese snakeroot grass with primers designed according to the transcriptome sequencing results of the Japanese snakeroot grass petal tissue, and then amplified by PCR.
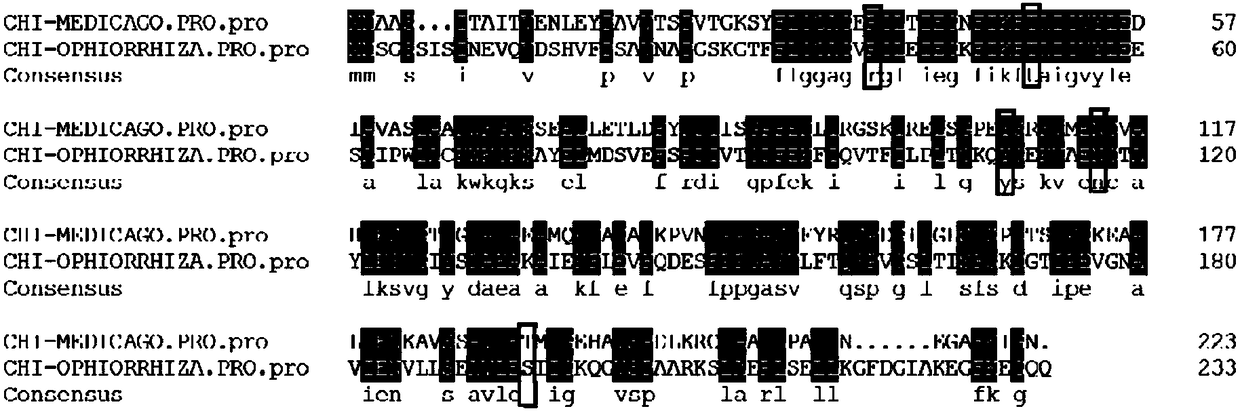
[0043] Compared with the chalcone isomerase CHI with known crystal structure of alfalfa, the results show that the CHI of snakeroot has a ubiquitous active site of chalcone isomerase, the results are as follows figure 1 As shown, CHI-MEDICAGO.PRO represents the amino acid sequence of alfalfa...
Embodiment 2
[0045] This example provides a study on the function of the chalcone isomerase gene, including further verification and determination through gene expression in vitro.
[0046] Construction of the recombinant plasmid: use the cDNA of the cloned full-length Japanese snakeroot chalcone isomerase OjCHI gene as a template, and perform PCR of the OjCHI open reading frame with primers containing BamH I and Hind III restriction sites respectively Amplify. The amplified DNA fragments were double-digested with two restriction enzymes, BamHI and HindIII, respectively, and ligated and cloned into the pET-32a vector. The thus constructed recombinant plasmid was named pET32-OjCHI, and introduced into the host cell Escherichia coli BL21(DE3) cells to prepare a large amount of soluble recombinant protein.
[0047] Prokaryotic expression of soluble recombinant chalcone isomerase protein;
[0048]The above-mentioned Escherichia coli was streak-inoculated in LB solid medium containing Amp, an...
Embodiment 3
[0052] Due to heterologous prokaryotic expression, it is easy to cause enzyme inactivation, mainly because the Japanese snakeroot chalcone isomerase belongs to the expression product of plant eukaryotic genes, and Escherichia coli belongs to the prokaryotic expression system. Different expression systems may be in the The folding process of the protein in the later stage is different, which leads to the inactivation of the protein, so the successful expression of the protein does not necessarily make the protein have the corresponding biological activity; therefore, the verification of the biological activity is required; in addition, due to the codon preference, it may also cause the protein to be inactivated. Expression failed, so sequence codon optimization can be performed based on the gene sequence of the cloned japonica chalcone isomerase provided by the present invention; so that the efficiency of prokaryotic expression is improved.
[0053] With naringenin chalcone as s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com