Method for determining roflumilast related substances by high performance liquid chromatography
A technology of high performance liquid chromatography and related substances, which is applied in the field of determination of related substances of roflumilast by high performance liquid chromatography, can solve the problems that the detection method needs to be improved, and achieve the improvement of drug safety, simple and fast method, and improvement of work efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 System suitability test of the detection method of the present invention.
[0047] Diluent: 0.005mol / L ammonium dihydrogen phosphate solution (adjust the pH value to 3.5 with phosphoric acid)-methanol-acetonitrile (41:31:28).
[0048] Blank solution: diluent.
[0049] Preparation of impurity reference substance stock solution: take appropriate amount of impurity A, impurity B, impurity C, impurity D, impurity E, impurity F and impurity G reference substance, weigh them accurately, dissolve them with diluent respectively and quantitatively dilute to make each 1ml Containing 500μg of the solution, shake well, that is.
[0050] Preparation of impurity localization solution: Take an appropriate amount of the above-mentioned impurity reference substance stock solution, quantitatively dilute with diluent respectively to make a solution containing 10 μg per 1 ml, shake well, and obtain.
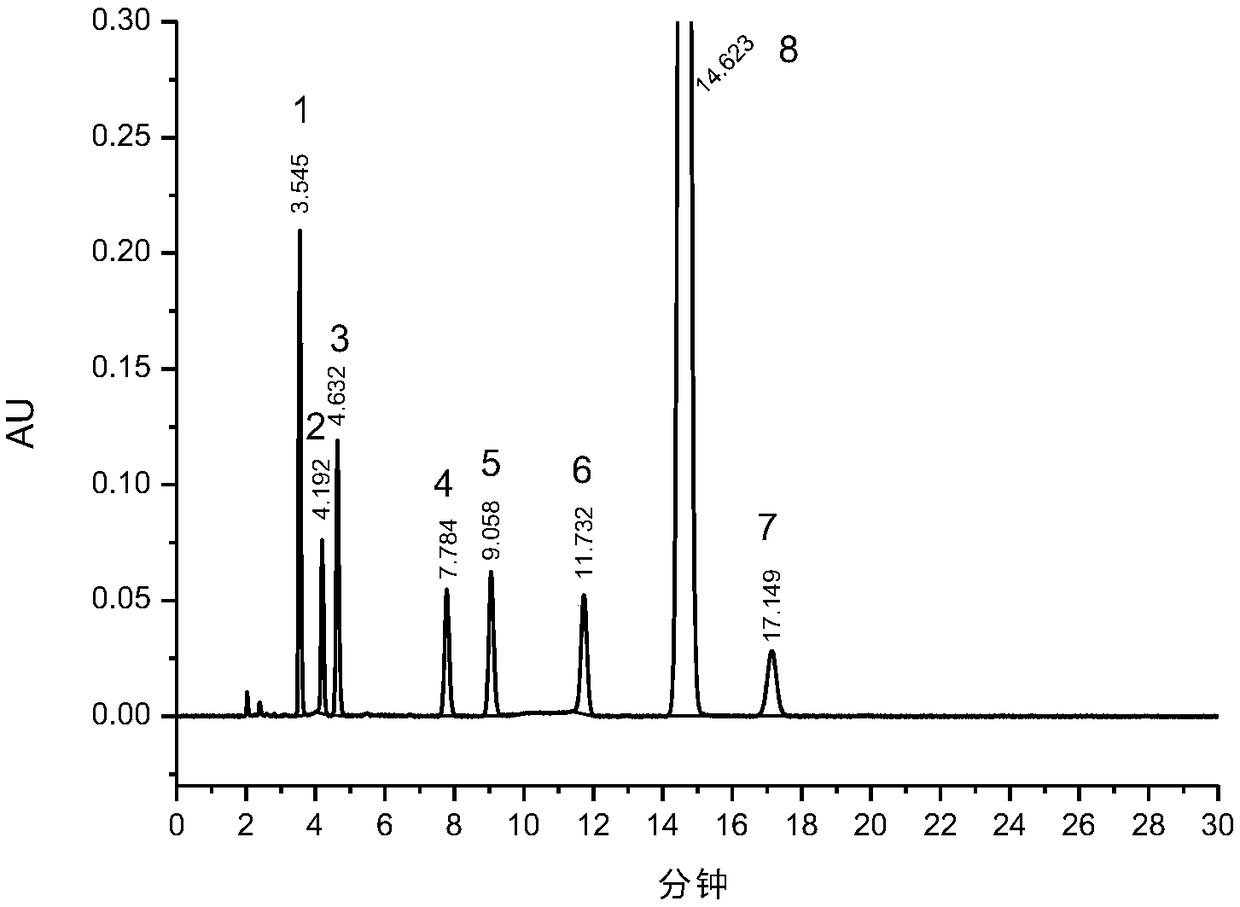
[0051]Preparation of system suitability solution: Take about 5 mg of roflumil...
Embodiment 2
[0056] Example 2 The specificity test of the detection method of the present invention.
[0057] The forced degradation test is to simulate the strong degradation conditions of strong acid, strong alkali, oxidation, light and high temperature to accelerate the destruction of roflumilast. Validity and applicability of analytical methods.
[0058] In order to better examine the specificity and stability of this method, forced degradation tests of acid, alkali, oxidation, light and high temperature were designed.
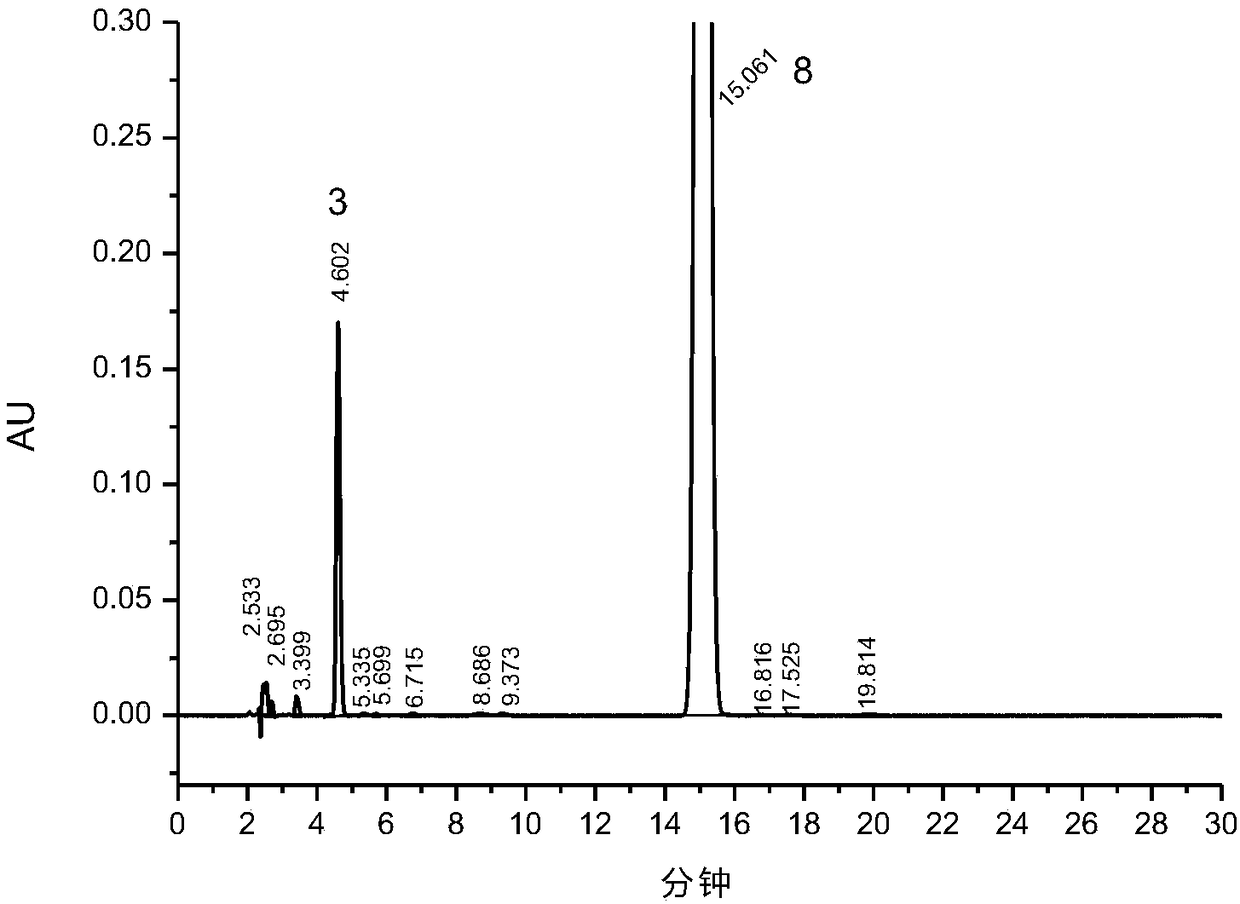
[0059] Acid-forced degradation test: take about 25mg of roflumilast, weigh it accurately, put it in a 50ml measuring bottle, add 5ml of acetonitrile and shake to dissolve, add 2ml of 1mol / L hydrochloric acid solution, shake well, put it in a water bath at 80°C for 3.5h to destroy , let it cool, add 1mol / L sodium hydroxide solution to neutralize, dilute to the mark with diluent, shake well, filter, take 10μl and inject it into the liquid chromatograph, record the chrom...
Embodiment 3
[0065] Example 3 Detection limit and quantification limit of the detection method of the present invention.
[0066] For related substances, the detection limit and quantitation limit are determined according to the signal-to-noise ratio method. Dilute the impurity reference substance stock solution of known concentration in Example 2 to a low-concentration sample, compare the measured signal with the baseline noise, and calculate the amount that can be reliably detected, with a signal-to-noise ratio of about 3:1, 10 :1 Calculate the detection limit and limit of quantification of roflumilast and each impurity respectively, and the results are shown in Table 3.
[0067] Table 3 The results of detection limit and quantitation limit
[0068]
[0069]
[0070] As can be seen from Table 3, the sensitivity of the method of the present invention is higher.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com