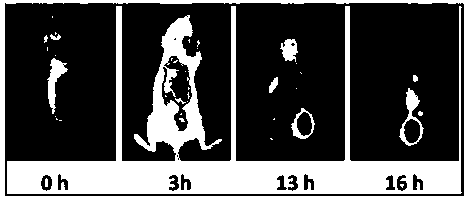
Organic matter and preparation method and application thereof
A technology of organic matter and reaction, applied in the field of biomedicine, can solve the problems of reduced application, difficult to degrade, difficult to control the size, etc., and achieve the effects of stable structure, easy synthesis, and simple control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The present invention has no special limitation on the preparation method of the organic compound with the structure of formula I, and it can be prepared according to the organic compound synthesis method well known to those skilled in the art. In the present invention, the organic compound of the formula I structure is preferably prepared according to the following method:
[0080] The compound of formula A and the compound of formula B are reacted to obtain the compound of formula I:
[0081]
[0082] R in Formula A 5 ~R 15 and W, Y, Z, R', R in Formula B 1 , R 2 , R 3 , R 4 and n and R in formula I 5 ~R 15 , W, Y, Z, R', R 1 , R 2 , R 3 , R 4 It is consistent with n and will not be repeated here.
[0083] In the present invention, the preparation method of the compound of formula B structure is preferably:
[0084] reacting the compound of the formula C structure and the compound of the formula D structure to obtain the compound of the formula B struc...
Embodiment 2
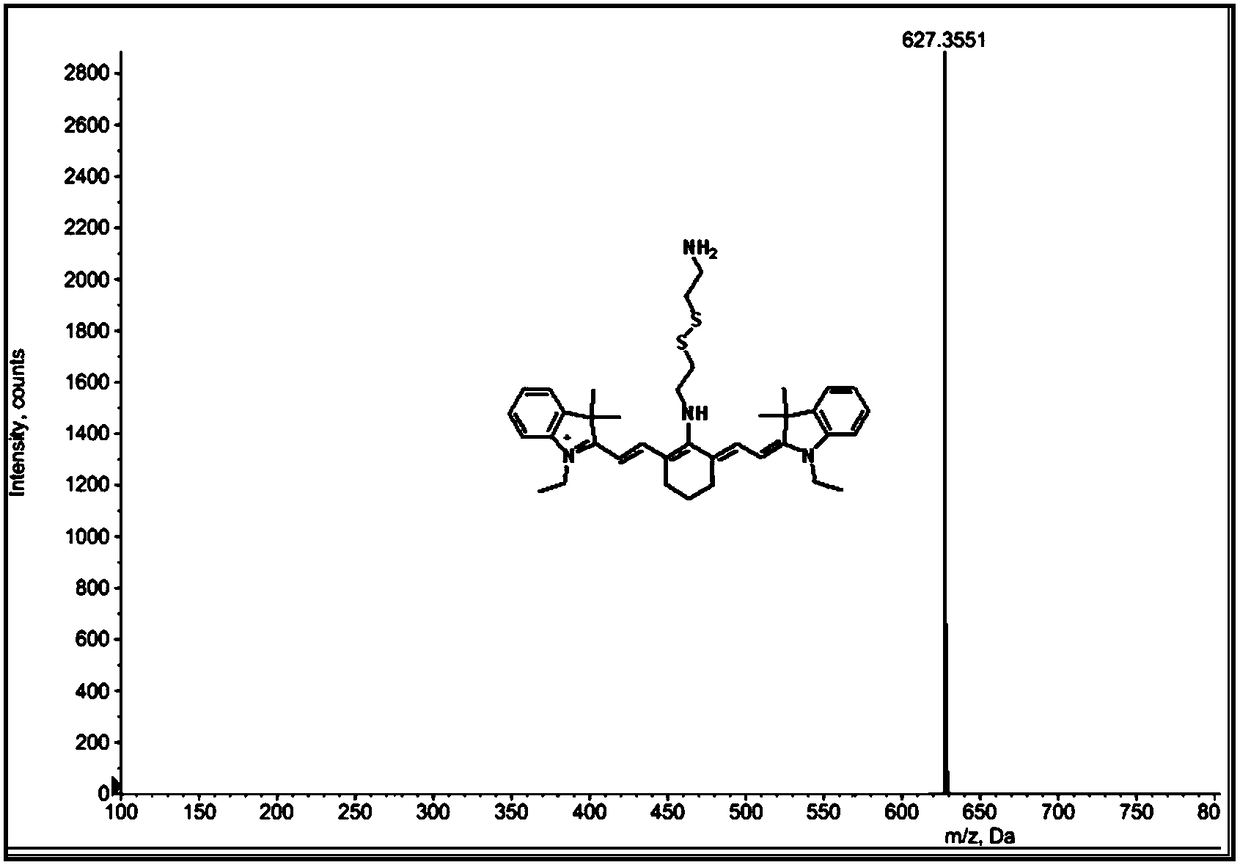
[0106] At room temperature, add cystamine dihydrochloride (162mg, 0.72mmol), 1.5mL methanol, 416μL triethylamine, and 2mL acetonitrile into a 25mL round bottom flask. The hydrochloride was completely dissolved. Then the compound of formula 3 (38.4mg, 0.6mmol) prepared in Example 1 was dissolved in 4mL of acetonitrile, and added dropwise to the above solution with a dropping funnel, about 1 drop / 20s, heated, and reacted under nitrogen protection at 35°C 4 Hour. The obtained reaction product was spun to remove the solvent, purified with a silica gel column, the sample was dissolved in dichloromethane, and the sample was wet-loaded, and the eluent was eluted with a dichloromethane:methanol gradient from 200:1 to 20:1, and the solvent was spun off After concentration, an indigo blue powdery solid was obtained, which was the compound of formula 2 (41.2 mg, 0.055 mmol), and the yield was 9.1%.
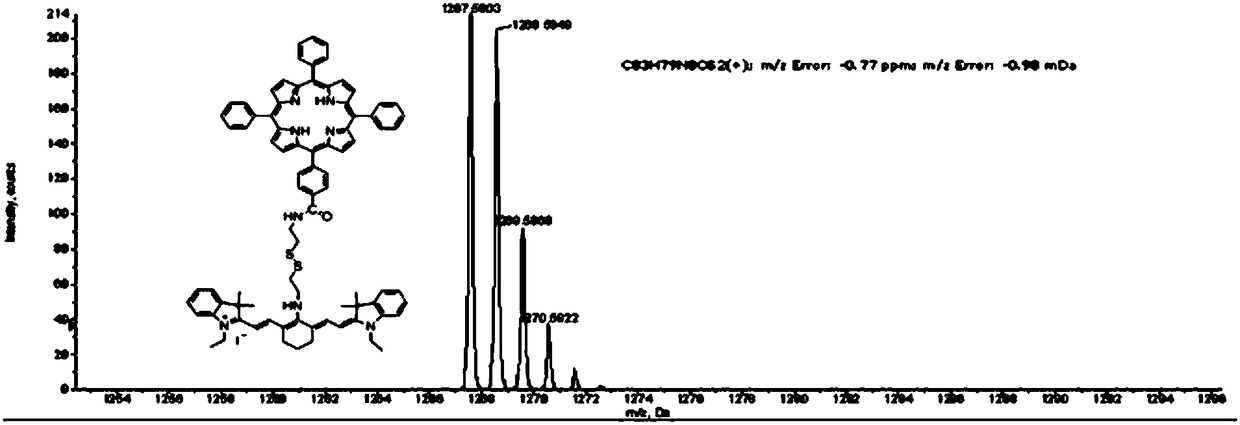
[0107] Under ice bath, the compound of formula 1 (5mmol, 3.29g), the compound of formu...
Embodiment 3
[0111] At room temperature, add cystamine dihydrochloride (324 mg, 1.44 mmol), 3 mL of methanol, 832 μL of triethylamine, and 4 mL of acetonitrile into a 50 mL round-bottomed flask. After stirring rapidly for about 0.5 hours, the solution becomes transparent and cystamine di The hydrochloride was completely dissolved. Then dissolve the compound of formula 3 (76.8mg, 1.2mmol) prepared in Example 1 in 8mL of acetonitrile, add dropwise to the above solution with a dropping funnel, about 1 drop / 20s, heat, and react under nitrogen protection at 35°C 4 hours. The obtained reaction product was spun to remove the solvent, purified with a silica gel column, the sample was dissolved in dichloromethane, and the sample was wet-loaded, and the eluent was eluted with a dichloromethane:methanol gradient from 200:1 to 20:1, and the solvent was spun off After concentration, an indigo blue powdery solid was obtained, which was the compound of formula 2 (78.78 mg, 0.105 mmol), yield: 8.7%.
[...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap