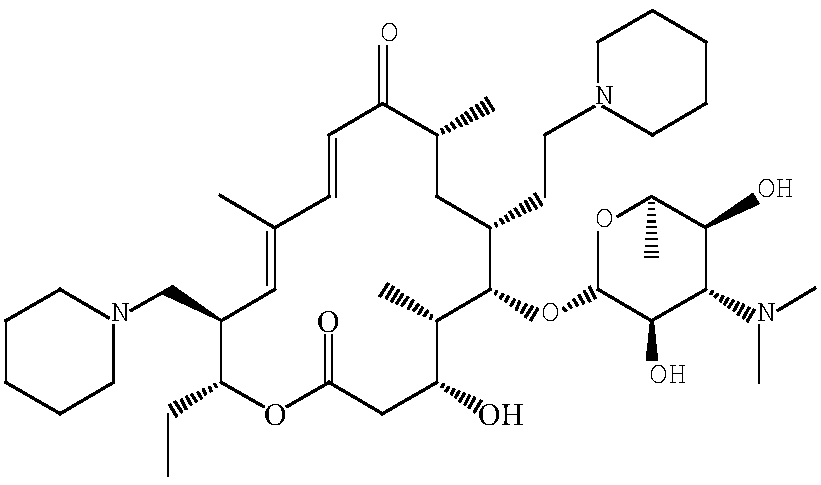
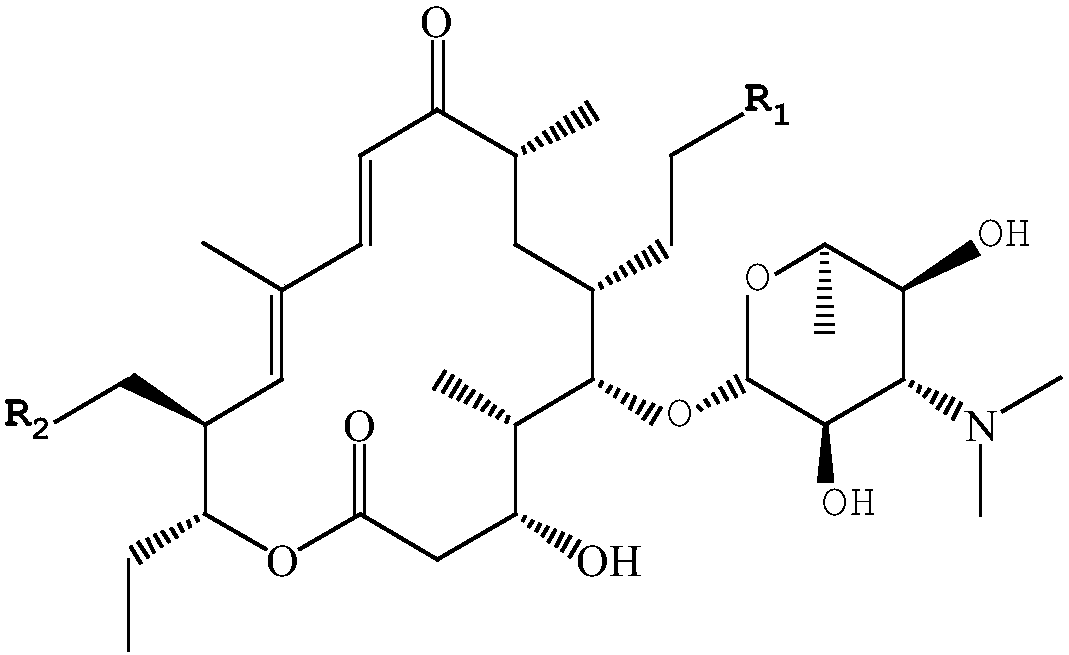
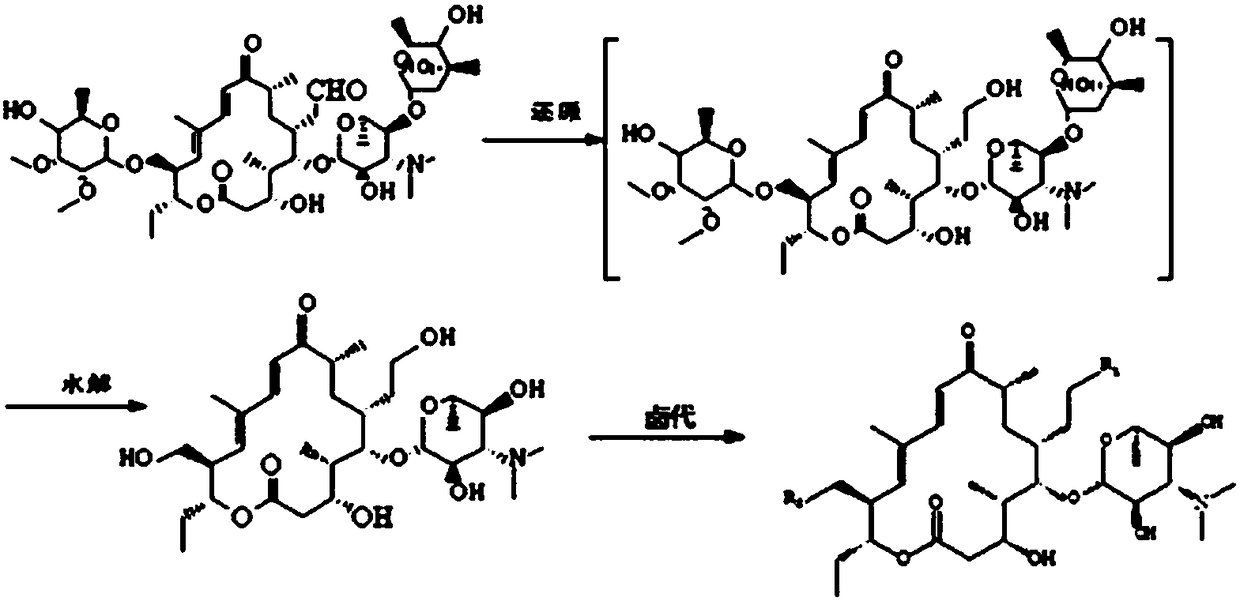
Synthetic method of 20,23-dipiperidine-5-O-carbomycin amine glycosyl-tildipirosin
A mycaminoglycosyl and tylonolide technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of cumbersome post-processing, complicated steps, and high three wastes, and save raw materials. cost, simplify the synthesis process, and reduce the effect of the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of synthetic method of 20,23-diiodo-5-O-mycaminosyl-tylonolide, the steps are as follows:
[0045] 1. In a 1000 ml three-necked flask, dissolve 300 g of tylosin phosphate in 700 ml of methanol, place the three-necked flask in an ice bath to cool, and at the same time feed nitrogen into the three-necked flask to make it in a nitrogen atmosphere, stir Slowly add 50 g of solid sodium borohydride, and stir for 3 hours to react. After the reaction is complete, stop feeding nitrogen, and cool the three-necked flask to 30° C. to obtain a mixed product.
[0046] 2. Add 0.5 mol / L sulfuric acid solution to the three-necked flask containing the mixed product, adjust the pH value of the system to 2, raise the temperature to 50°C, and keep it warm for 3 hours to obtain the crude intermediate. Dissolve the crude intermediate in 1000 ml of water Dilute with 1400 ml of dichloromethane, add 5mol / L sodium hydroxide solution under stirring to adjust the pH to 6.5, let stand to sepa...
Embodiment 2
[0052] A kind of synthetic method of 20,23-diiodo-5-O-mycaminosyl-tylonolide, the steps are as follows:
[0053] 1. In a 1000 ml three-necked flask, dissolve 300 grams of tylosin phosphate in 700 ml of ethanol, place the three-necked flask in an ice bath to cool, and at the same time, feed nitrogen into the three-necked flask to make it in a nitrogen atmosphere, and stir Slowly add 80g of zinc amalgam under low temperature, stir and react for 8 hours, stop feeding nitrogen after the reaction is completed, and cool the three-necked flask to 30°C to obtain a mixed product.
[0054] 2. Add 40wt% hydrobromic acid solution to the three-necked flask containing the mixed product, adjust the pH value of the system to 2, heat up to 50°C, and heat-preserve and hydrolyze for 8 hours to obtain the crude intermediate, which is mixed with 1000 ml of water Dilute with 1400 ml of dichloromethane, add 5mol / L sodium hydroxide solution under stirring to adjust the pH to 6.5, let stand to separat...
Embodiment 3
[0057] A kind of synthetic method of 20,23-diiodo-5-O-mycaminosyl-tylonolide, the steps are as follows:
[0058] 1. In a 1000 ml three-necked flask, dissolve 300 grams of tylosin phosphate in 700 ml of butanone, place the three-necked flask in an ice bath to cool, and simultaneously feed nitrogen into the three-necked flask to make it in a nitrogen atmosphere, Slowly add 50 g of aluminum isopropoxide under stirring, and react with stirring for 10 hours. After the reaction is completed, stop feeding nitrogen, and cool the three-necked flask to 30° C. to obtain a mixed product.
[0059] 2. Add 85wt% formic acid solution to the three-necked flask containing the mixed product, adjust the pH value of the system to 2, heat up to 50°C, and heat-preserve and hydrolyze for 24 hours to obtain the crude intermediate. The crude intermediate is mixed with 1000 ml of water and 1400 Dilute with milliliter of dichloromethane, add 5mol / L sodium hydroxide solution under stirring to adjust the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com