Recombinant virus silencing T1 protein, and construction method and application thereof
A technology of recombinant virus and construction method, which is applied in the field of biomedicine, can solve the problems that affect the treatment effect of TrkB atherosclerosis, it is not easy to obtain high titer virus, and the expression level is low, so as to protect the integrity of the endothelial barrier and inhibit plaque The area of the plaque, the lipid and macrophage content in the plaque, and the effect of promoting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Construction of a recombinant virus for silencing T1 protein
[0040] 1. Design and synthesize the shRNA sequence based on the mouse T1 mRNA (NM_008745), and introduce a BamHI restriction site at the 5' end and a HindⅢ restriction site at the 3' end. The sequence is as follows:
[0041] mT1-shRNA-F (SEQ ID NO.1, the underline is the HindⅢ restriction site):
[0042] 5'-GATCCGCAGTTTCTCTAGTGTGAATAGTTCAAGAGACTATTCACACTAGAGAACTGCTTTTTTAGATCTA-3',
[0043] mT1-shRNA-R (SEQ ID NO.2, the underline is the BamHI restriction site):
[0044] 5'- AGCTT AGATCTAAAAAAGCAGTTTCTAGTGTGAATAGTCTCTTGAACTATTCACACTAGAGAACTGCG-3';
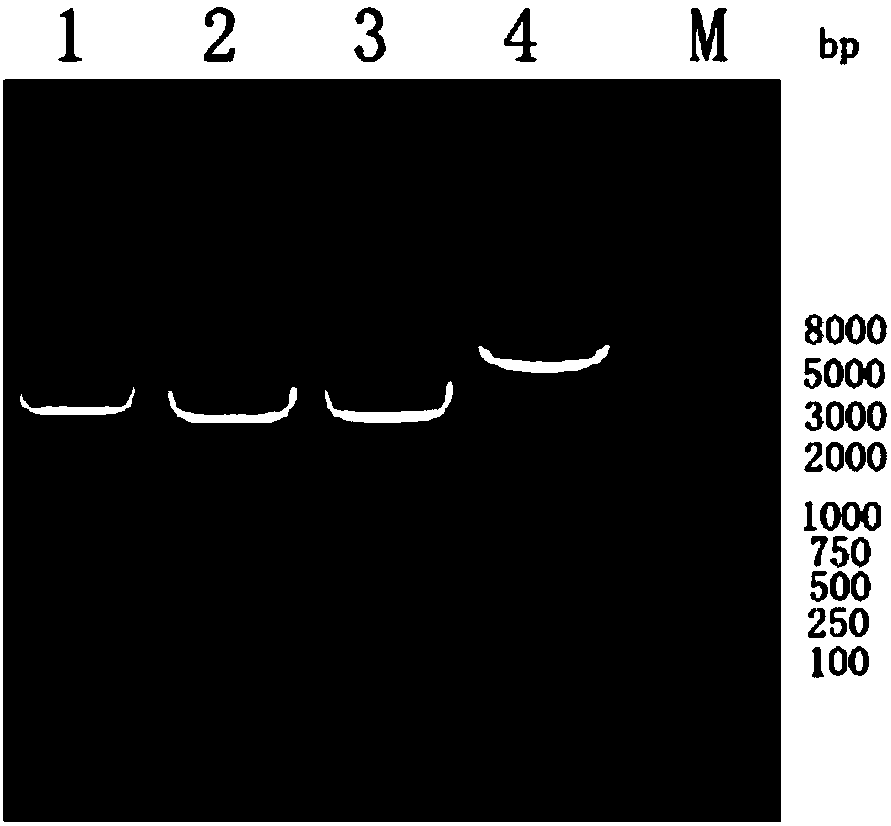
[0045] 2. Fully dissolve the mT1-shRNA-F and mT1-shRNA-R fragments in step 1 in 100 μL annealing buffer respectively, take 5 μL of each solution and add them to 5 μL 10× annealing buffer, add sterilized water to 50 μL, Mix well, anneal at 100°C for 2 minutes, cool naturally to room temperature, and dilute 100 times with sterile water to obtain the m...
Embodiment 2
[0058] Example 2: Verification of in vivo infection efficiency and silencing efficiency of recombinant virus
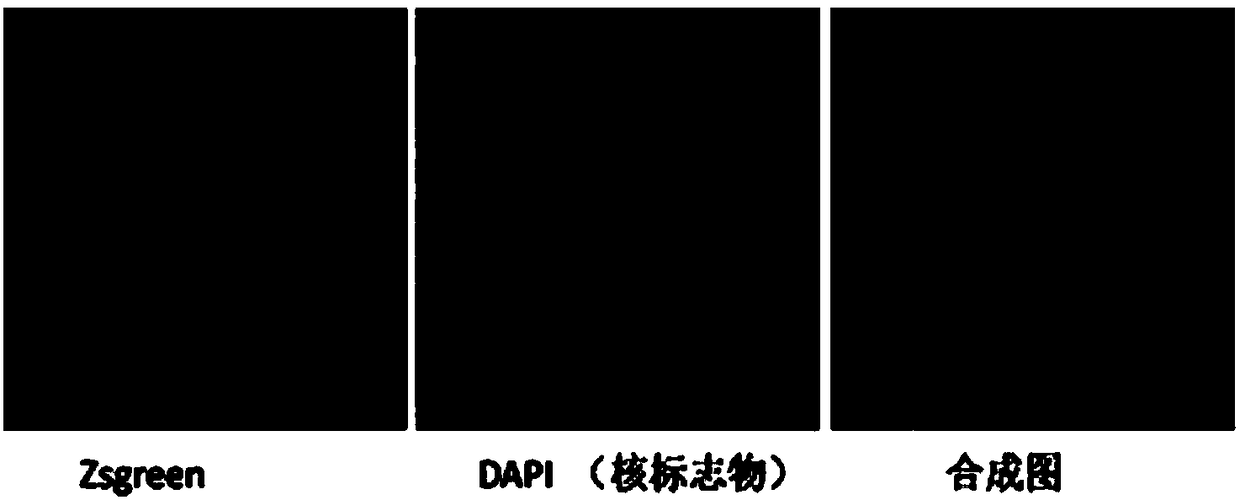
[0059] Sixty 8-week-old C57BL / 6 mice were selected and divided into two groups. The control group was injected with a recombinant virus containing scrambled shRNA (rAAV9-ZsGreen-ShRNA-mScramble) through the tail vein of the mice, and the experimental group was injected with The recombinant virus for silencing the T1 protein constructed in Example 1 was injected through the mouse tail vein with a disposable syringe, and the injection dose was 7×10 10 vg / g body weight and 20 μL / g body weight, fed with normal diet for 16 weeks;
[0060] Preparation of cryosections from mouse aorta
[0061] Immunofluorescence staining, observing the expression of reporter gene ZsGreen, verifying the infection efficiency; detecting the expression of T1 protein, verifying the silencing efficiency: frozen sections of aortic root were fixed with 4% paraformaldehyde, soaked in PBS, blocked wi...
Embodiment 3
[0063] Example 3: Application of recombinant virus in improving endothelial function in mice with atherosclerosis
[0064] To establish an animal model of endothelial injury: select 60 8-week-old ApoE knockout mice, divide them into 2 groups, and feed them normally for 16 weeks;
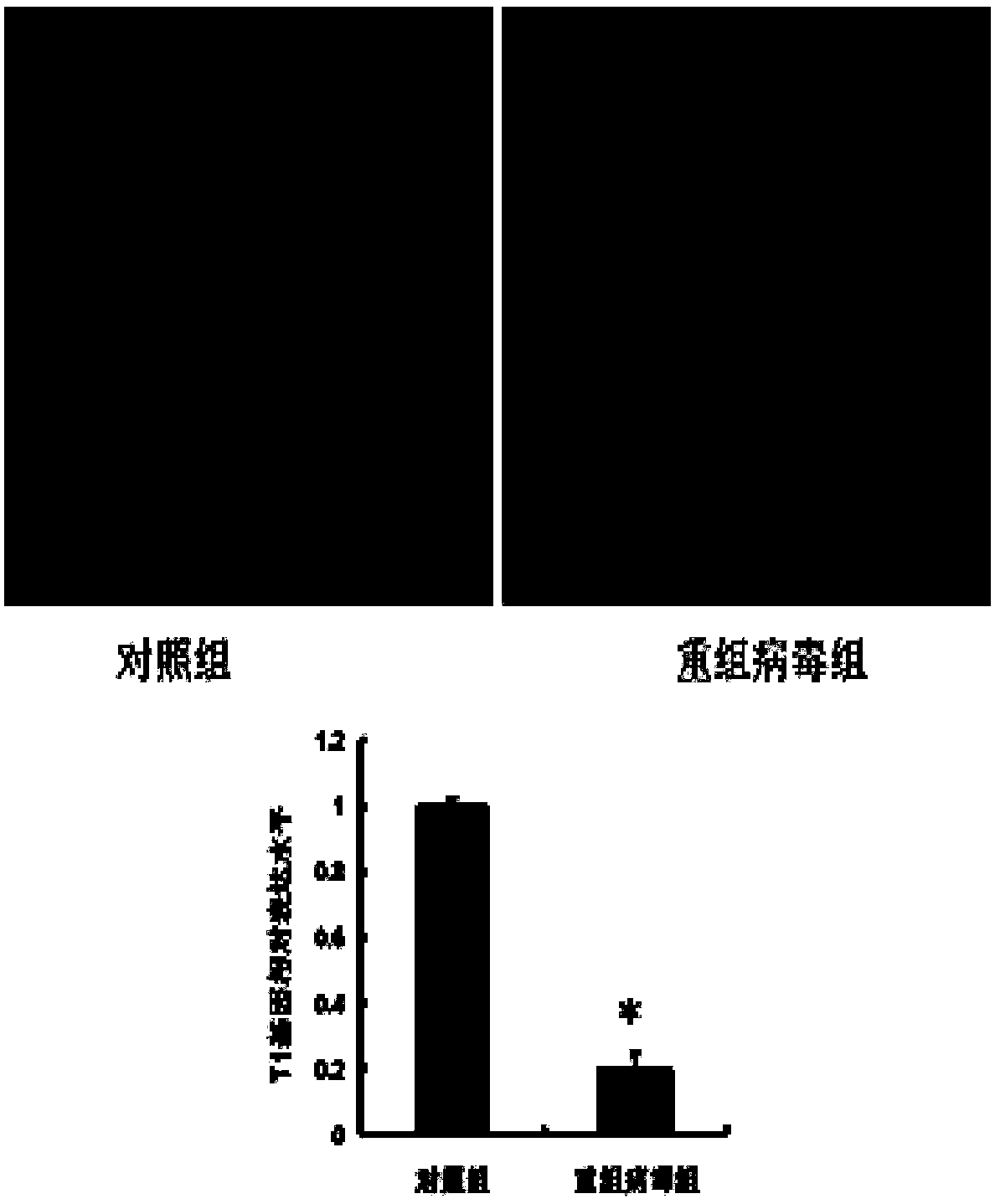
[0065] At the beginning of modeling, the mice were simultaneously injected with the recombinant virus through the tail vein of the mouse with a disposable syringe, the control group was injected with the recombinant virus containing scrambled shRNA (rAAV9-ZsGreen-ShRNA-mScramble), and the experimental group was injected with the recombinant virus constructed in Example 1. Recombinant virus for silencing T1 protein, the injection dose is 7×10 1 0vg / g body weight and 20μL / g body weight;
[0066] After 16 weeks of normal feeding, Evans blue was dissolved in 0.2ml PBS according to the dose of 20mg / kg body weight, injected through the tail vein of the mouse, and anesthetized by intraperitoneal injection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com