Compound paracetamol tablet and preparation method thereof
A technology of acetaminophen tablets and compound recipes, which is applied in the direction of medical formulas, medical preparations containing active ingredients, and medical preparations containing active ingredients. Reduce, poor dissolution rate and other problems, to achieve the effect of accelerating disintegration and dissolution time limit, improving drug stability, and accelerating dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The present invention also provides a preparation method of compound paracetamol tablet, comprising the following steps:
[0026] S1, pretreatment;
[0027] Acetylsalicylic acid, caffeine, acetaminophen, stabilizers, glidants, fillers, and cellulose disintegrating agents are respectively passed through 80-100 mesh sieves, and the binder is respectively passed through 60-80 mesh sieves.
[0028] Prepare the first mixture binder: 3-5% filler (the filler used this time accounts for 3-5% of the mass ratio of all fillers) mixed with water to prepare starch with a mass fraction of 15-20% pulp.
[0029] Preparation of the second mixture binder: the inclusion binder is mixed with a wetting agent to obtain an inclusion binder solution with a concentration of 5-7%, that is, the second mixture binder.
[0030] S2, granulation;
[0031] S2.1, preparing the first mixture;
[0032] Put the weighed acetaminophen, caffeine, and cellulose disintegrating agent into the GHL-250 high-e...
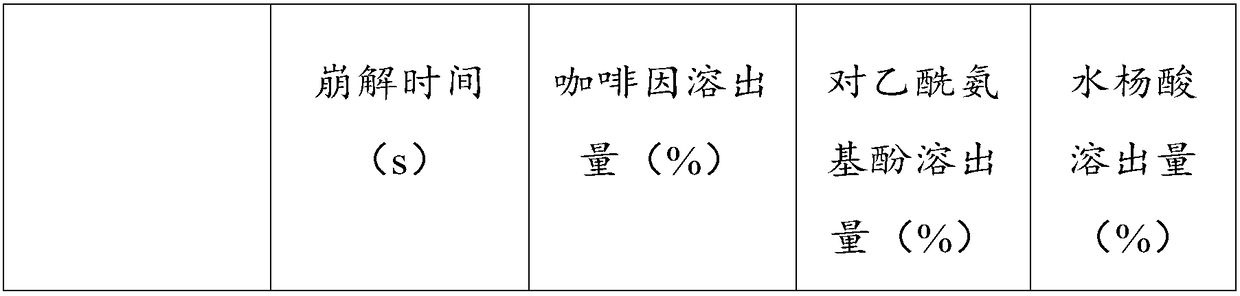
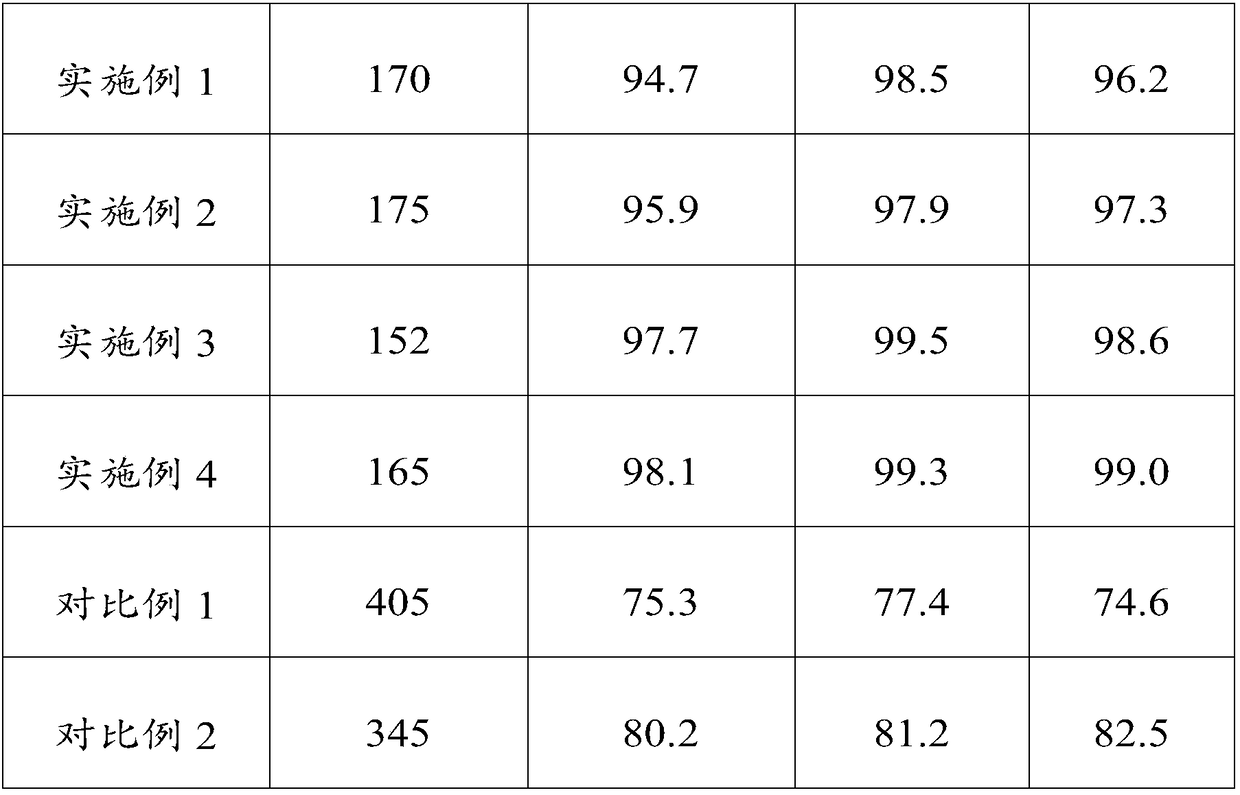
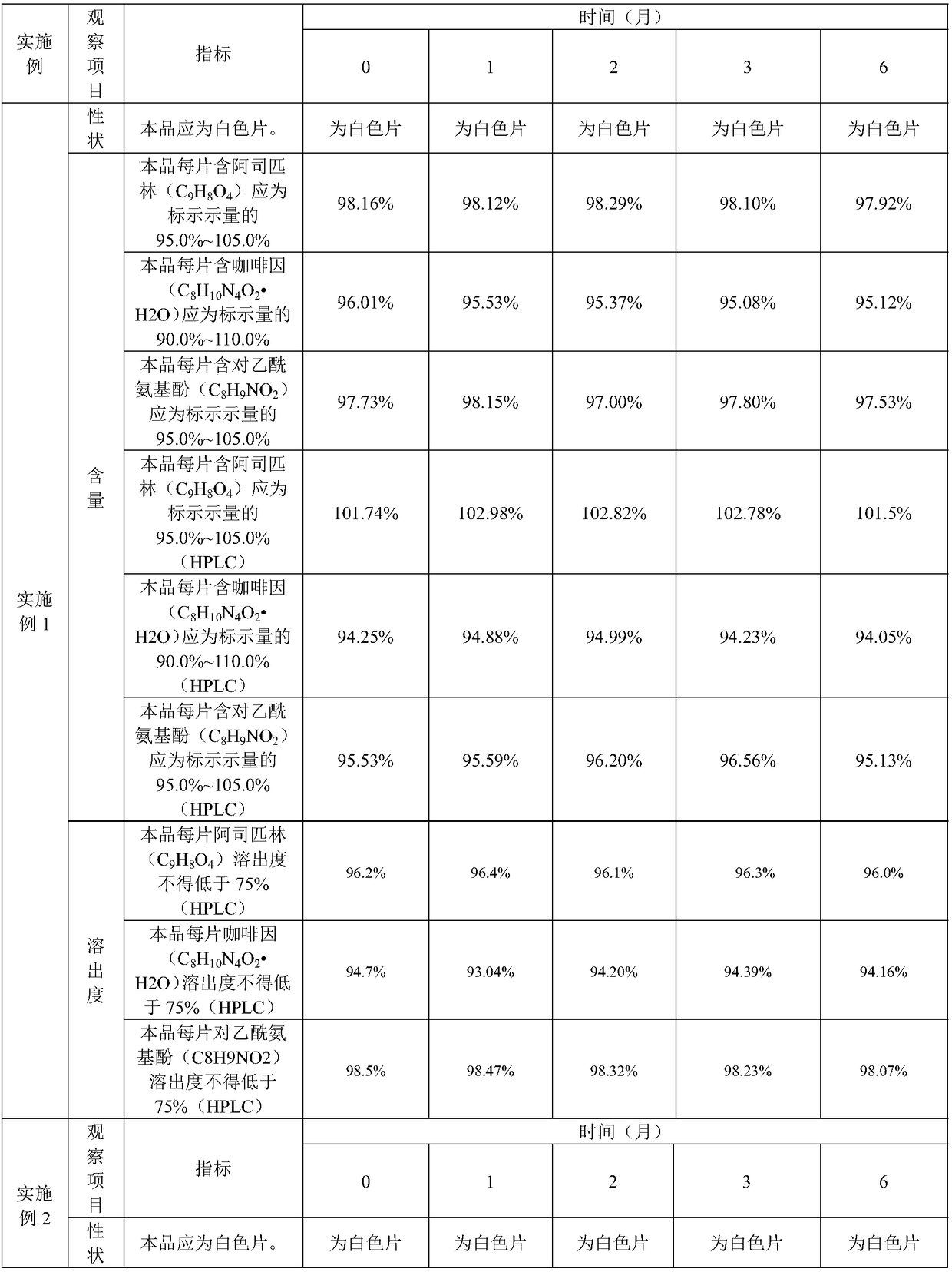
Embodiment 1
[0046] This embodiment provides a kind of compound paracetamol sheet (300,000 tablets), and it is made of various raw materials, and multiple raw materials are 65Kg paracetamol, 35Kg acetylsalicylic acid, 8Kg caffeine, 2Kg compound Disintegrant, 0.5Kg ethyl cellulose and 30.5Kg auxiliary materials. Among them, 2Kg of compound disintegrating agent is 1Kg of hydroxypropyl cellulose and 1Kg of polacrilin potassium; 30.5Kg of auxiliary materials are 0.5Kg of citric acid, 15Kg of starch, 5Kg of talcum powder, 5Kg of pregelatinized starch and 10Kg of ethanol.
[0047] The present embodiment provides a kind of preparation method of compound paracetamol tablet, comprises the following steps:
[0048] S1, pretreatment;
[0049] Acetylsalicylic acid, caffeine, acetaminophen, citric acid, talcum powder, starch, and hydroxypropyl cellulose were respectively passed through a 80-mesh sieve, and the pregelatinized starch was respectively passed through a 60-mesh sieve.
[0050] Preparation...
Embodiment 2
[0067] The composition of the compound paracetamol tablet provided in this example is basically the same as that of the compound paracetamol tablet provided in Example 1, the difference is that the usage amount of each component changes, specifically, various raw and auxiliary materials are 70Kg paracetamol Phenol, 40Kg acetylsalicylic acid, 12Kg caffeine, 4Kg compound disintegrant, 1Kg ethyl cellulose and 54Kg auxiliary materials. Among them, the 4Kg composite disintegrant is 2Kg hydroxypropyl cellulose and 2Kg polacrilin potassium; the 54Kg auxiliary materials are 1Kg citric acid, 25Kg starch, 7Kg talcum powder, 6Kg pregelatinized starch and 15Kg ethanol.
[0068] The preparation method of the compound acetaminophen tablet provided in this example is basically the same as the preparation method of the compound acetaminophen tablet provided in Example 1, except that the operating conditions are changed. Pretreatment and sieving, respectively passing through 100 mesh sieve and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com