Ginsenoside-insulin nano gel as well as preparation method and application thereof
A technology of ginsenoside and nanogel, which is applied in the direction of medical formula, medical preparations with non-active ingredients, and medical preparations containing active ingredients. release, the effect of small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Weigh 5g of total ginsenosides, add acetic acid solution with pH 1.8 to 25ml, decompose at 90°C for 4h, leave the reactant at room temperature for 12h, filter with a 0.45μm filter membrane to remove insoluble matter, and use 10% sodium carbonate for the filtrate Neutralize to a pH of 8, then filter with a 2 μm filter membrane, and collect the precipitate; add the precipitate to 20 mL of absolute ethanol, heat to 60°C to dissolve it, leave it to cool at 4°C for 2 hours, and then filter it with a 2 μm filter paper. The filtrate was concentrated under reduced pressure and dried to obtain ginsenoside gel powder;
[0036] 2) Take insulin and ginsenoside gel powder, add secondary water, so that the final concentration of ginsenoside in the solution is 1~40 mg / mL, and the concentration of insulin is 1~25U / mL, and ultrasonically shake for 15 minutes to obtain ginsenoside / Insulin nanogel nanoparticle solution;
[0037] 3) Dissolve 0.5g of carbomer, 0.35g of gelatin, 0.5mg o...
Embodiment 2
[0038] Embodiment 2 Characterization of ginsenoside-insulin nanogel:
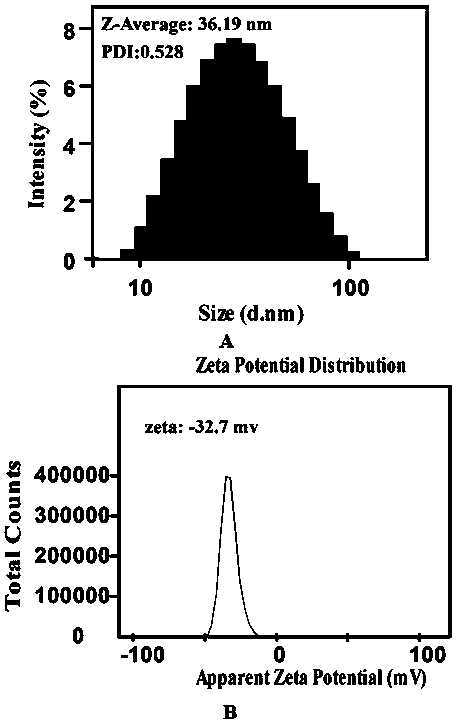
[0039] 1) Use dynamic light scattering (DLS) to measure the particle size (size, nm) and potential (zeta potential, mv) of the nanogel, the results are as follows figure 1 shown.
[0040] Depend on figure 1 It can be seen that the nanogel prepared by the present invention has a small particle size and a negative potential.
[0041] 2) The three-dimensional structure of the nanogel was determined by atomic force microscopy (AFM), and the results are as follows figure 2 shown.
[0042] Depend on figure 2 It can be seen that the ginsenoside / insulin prepared by the present invention is in the form of gel.
Embodiment 3
[0043]Example 3 In vitro percutaneous absorption test:
[0044] 1) In vitro percutaneous absorption experiment in mice
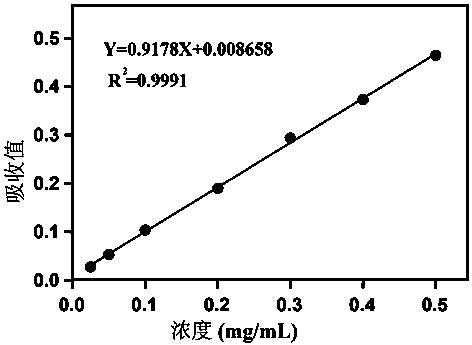
[0045] At room temperature, prepare an appropriate amount of BCA working solution by adding 50 volumes of BCA reagent A and 1 volume of BCA reagent B (50:1), and mix well. BCA working solution is stable at room temperature for 24 hours. Completely dissolve the protein standard, take 10 μL and dilute to 100 μL to make the final concentration 0.5mg / ml, and dilute the diluted standard (0.5mg / ml) at 0, 2, 4, 6, 8, 12, 16 , 20 μL were added to EP tubes, and PBS was added to make up to 20 μL. Add 200 μL of BCA working solution to each tube, place it at 37°C for 30 minutes, and draw the standard curve of protein quantification according to the absorbance value at the wavelength between 562nm and the results are as follows: image 3 shown.
[0046] Depend on image 3 It can be seen that the linearity of the protein content determined by the BCA method is good. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com