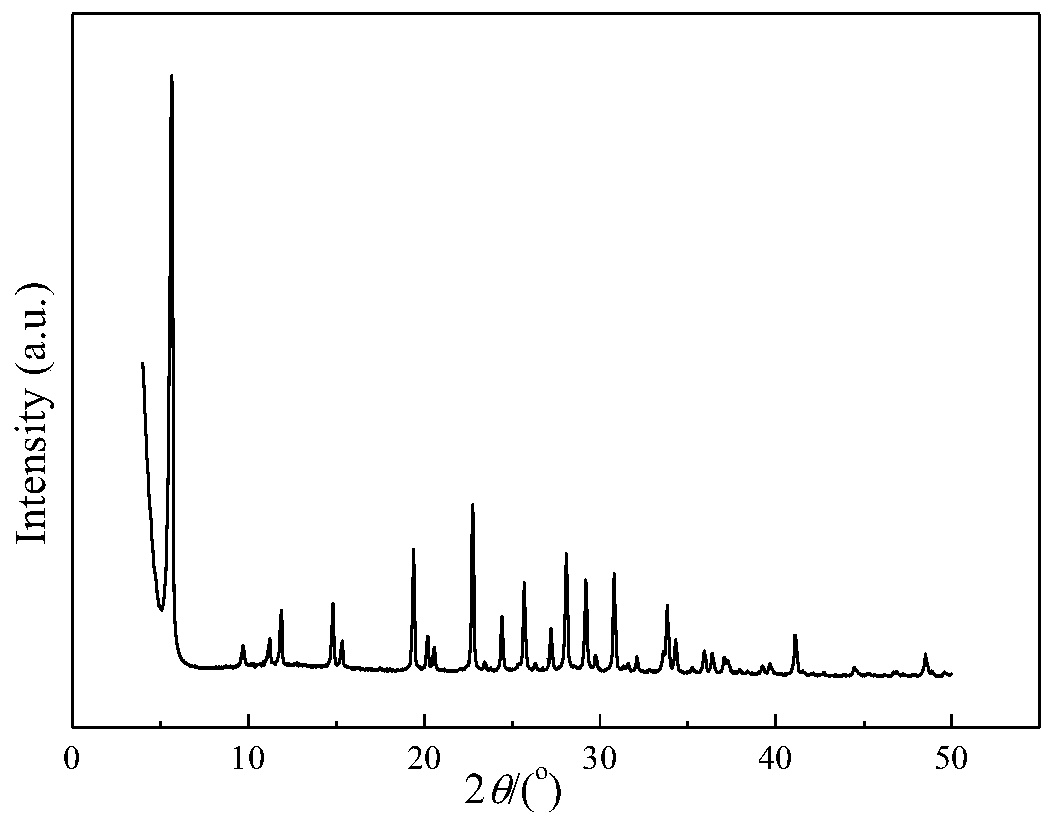
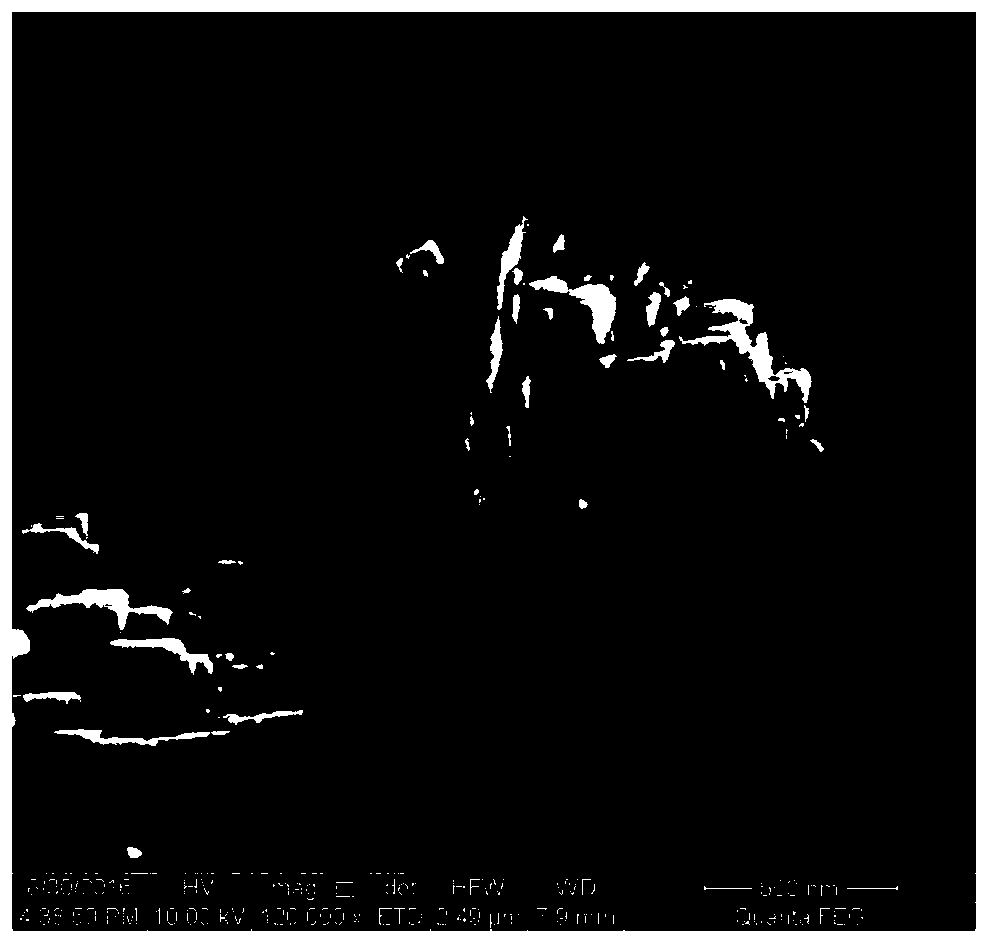
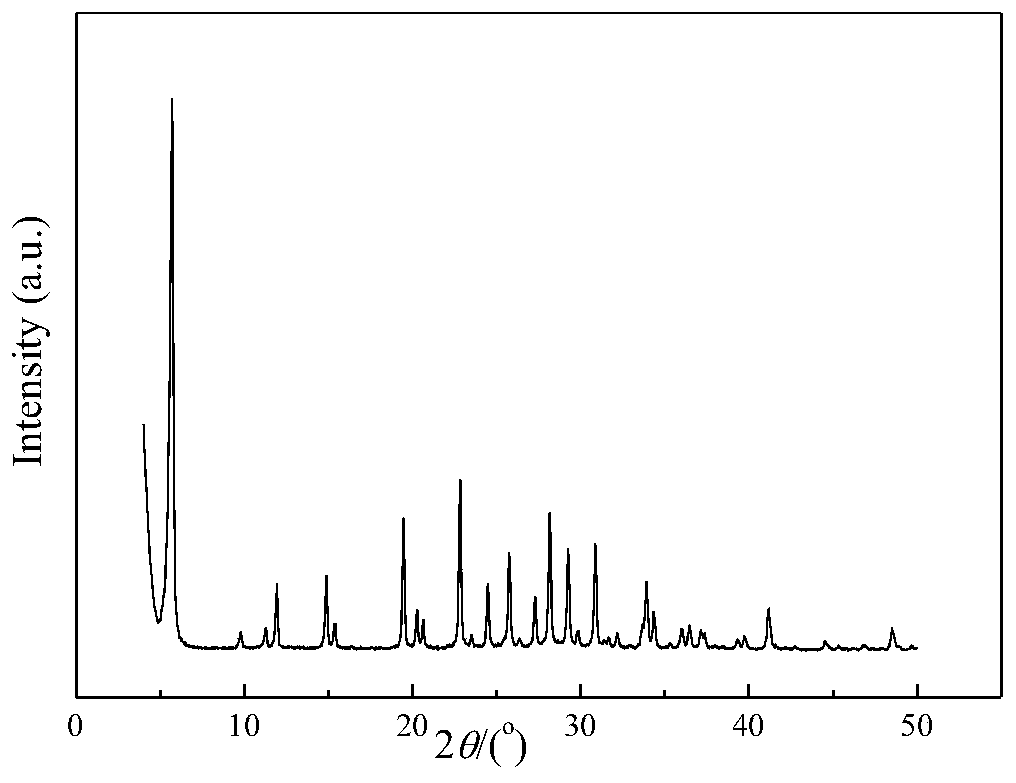
A kind of CE isomorphous substituted LTL molecular sieve and preparation method thereof
A technology of isomorphic substitution and molecular sieve, which is applied in the field of inorganic chemical synthesis, can solve the problems that the placement of heteroatoms is not explained, and the yield of aromatic hydrocarbons from alkane aromatization is not very high, and achieves good industrialization prospects, high crystallinity, and improved liquid crystallinity. The effect of receiving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Take 100.3g of KOH and 45.3g of aluminum hydroxide into 1300g of deionized water, stir evenly, transfer to a three-necked flask and react at 90°C for 12h, and cool to room temperature to obtain clear KAlO 2 Solution, K in the aluminum source solution 2 O:Al 2 O 3 The molar ratio is 2.56:1. Slowly add 668g of silica sol (SiO 2 The content is 30wt%), and the initial sol is obtained after strong stirring. Take 12.85g Ce(NO 3 ) 3 ·6H 2 O was dissolved in 200 g of deionized water to prepare a cerium source solution. The cerium source solution was slowly dropped into the sol and continued to stir vigorously. After aging at 20°C for 5 hours, it was transferred to a dynamic kettle and reacted at 150°C for 48 hours. After the crystallization is completed, the reactant is cooled to room temperature, filtered, washed with deionized water to neutrality, dried at 130°C for 6 hours, and calcined at 500°C for 4 hours to obtain Ce-containing LTL zeolite.
[0048] Among them, the amount o...
Embodiment 2
[0051] Add 100.3g KOH and 45.3g aluminum hydroxide to 1200g deionized water, stir well, transfer to a three-necked flask and react at 100°C for 24h. After cooling to room temperature, clear KAlO is obtained. 2 Solution, K in the aluminum source solution 2 O:Al 2 O 3 The molar ratio is 2.56:1. Slowly add 668g of silica sol (SiO 2 The content is 30wt%), and the initial sol is obtained after strong stirring. Take 64.25g Ce(NO 3 ) 3 ·6H 2 O was dissolved in 300 g of deionized water to prepare a cerium source solution. The cerium source solution was slowly added dropwise to the sol and continued to stir vigorously. After aging at 20°C for 5 hours, it was transferred to a dynamic kettle and reacted at 180°C for 48 hours. After the completion of crystallization, the reactant was cooled to room temperature, filtered, washed with deionized water to neutrality, dried at 100°C for 12h and calcined at 450°C for 8h to obtain Ce-containing LTL zeolite.
[0052] Among them, the amount of alumi...
Embodiment 3
[0055] Add 200.6g KOH and 45.3g aluminum hydroxide to 1100g deionized water, stir evenly, transfer to a three-necked flask, react at 90°C for 12h, and cool to room temperature to obtain clear KAlO 2 Solution, K in the aluminum source solution 2 O:Al 2 O 3 The molar ratio is 5.12:1. Slowly add 334g silica sol (SiO 2 The content is 30wt%), and the initial sol is obtained after strong stirring. Take 64.25g Ce(NO 3 ) 3 ·6H 2 O was dissolved in 400 g of deionized water to prepare a cerium source solution. The cerium source solution was slowly dropped into the sol and continued to stir vigorously. After aging at 20°C for 5 hours, it was transferred to a dynamic kettle and reacted at 150°C for 48 hours. After the completion of crystallization, the reactant was cooled to room temperature, filtered, washed with deionized water to neutrality, dried at 85°C for 22h and calcined at 580°C for 4h to obtain Ce-containing LTL zeolite.
[0056] Among them, the amount of aluminum hydroxide is Al ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com