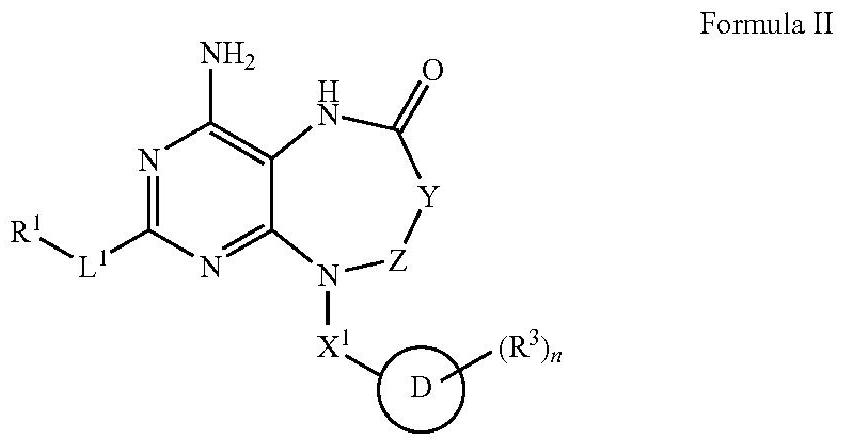
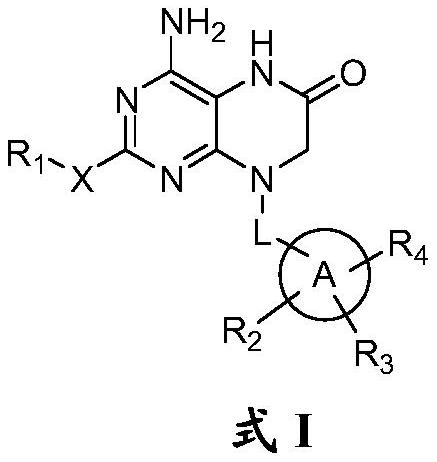
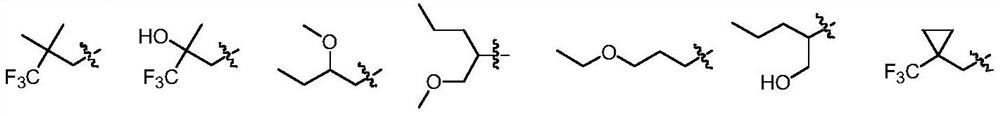
Dihydropteridinone derivatives, preparation method and use thereof
A kind of technology of tetrahydropyridine and piperidine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0312] Preparation 1: N-(6-amino-2-methylsulfonyl-5-nitropyrimidin-4-yl)-N-(3-(pyrrolidine-1-methyl)benzyl)glycine ethyl ester (intermediate Preparation of body 1)
[0313]
[0314] The first step: the preparation of 3-(pyrrolidine-1-methyl)benzonitrile
[0315] 3-cyanobenzaldehyde (60g, 0.458mol) was added to a three-necked flask, THF (500mL) was added to dissolve, pyrrole and acetic acid were added; ice-water bath was cooled, and NaBH(OAc) was added 3 (145g, 0.687mol), after addition, slowly rise to room temperature and stir overnight (about 20 hours); TLC detects that the reaction is complete, the reaction solution is added to 1.5L water, and NaHCO 3 Solution Adjust the pH value of the aqueous solution to 8-9; add EA (500mL×1, 300mL×2) to extract the aqueous layer, combine the EA, wash with saturated brine, and dry over anhydrous sodium sulfate. Concentration of EA gave the title compound (62 g, yield 73%).
[0316] The second step: the preparation of 3-(pyrrolidine-1...
preparation example 2
[0324]Preparation Example 2: Preparation of 3-(4-amino-2-butoxy-6-oxo-6,7-dihydropteridin-8(5H)-yl)methyl-benzaldehyde (Intermediate 2) :
[0325]
[0326] The first step of preparation of N-(3-cyanobenzyl) glycine ethyl ester
[0327] Add m-cyanobenzaldehyde (80g, 0.61mol) and glycine ethyl ester hydrochloride (94g, 0.67mol) into a 2L three-necked flask, add 1.2L methanol, triethylamine and NaBH 3 CN, stirred at room temperature for 6 hours; TLC detected that the reaction was complete, added 4L of water and EA (1L x 1, 500mL x 2) to the reaction solution to extract the aqueous layer, combined EA, washed with saturated brine, and dried over anhydrous sodium sulfate. Concentration of EA yielded 112 g of the product with a yield of 84%.
[0328] The second step: the preparation of N-(6-amino-2-methylthio-5-nitropyrimidin-4-yl)-N-(3-cyanobenzyl)glycine ethyl ester
[0329] Add (3-cyanobenzyl)glycine ethyl ester and 6-amino-5-nitro-2-methylthio-4-chloropyrimidine into a 2L t...
preparation example 3
[0338] Preparation Example 3: Preparation of 6-chloro-2-methylthio-5-nitropyrimidin-4-amine (Intermediate 3)
[0339]
[0340] The raw material 5-nitro-2-methylthio-4,6-dichloropyrimidine (120g, 0.5mol) was added into a three-necked flask and dissolved in 600mL THF, then triethylamine and ammonia methanol (86mL, 0.6mol) were added, and the The reaction was stirred for 1 hour; TLC detected that the reaction was complete, and the reaction solution was added to 2L of water, filtered with suction, and the filter cake was successively washed with purified water (500mL×2) and petroleum ether (400mL) and dried to obtain 95g of the target product, with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com