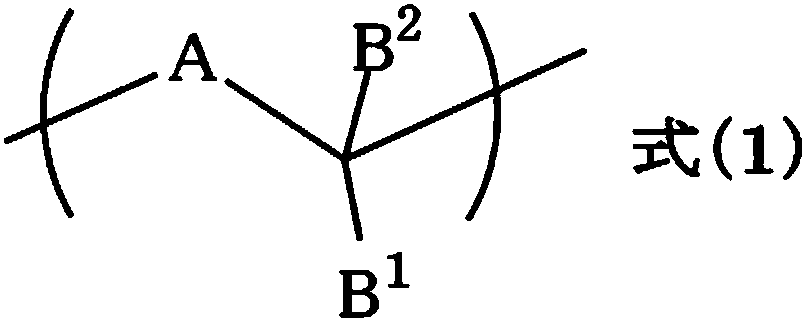
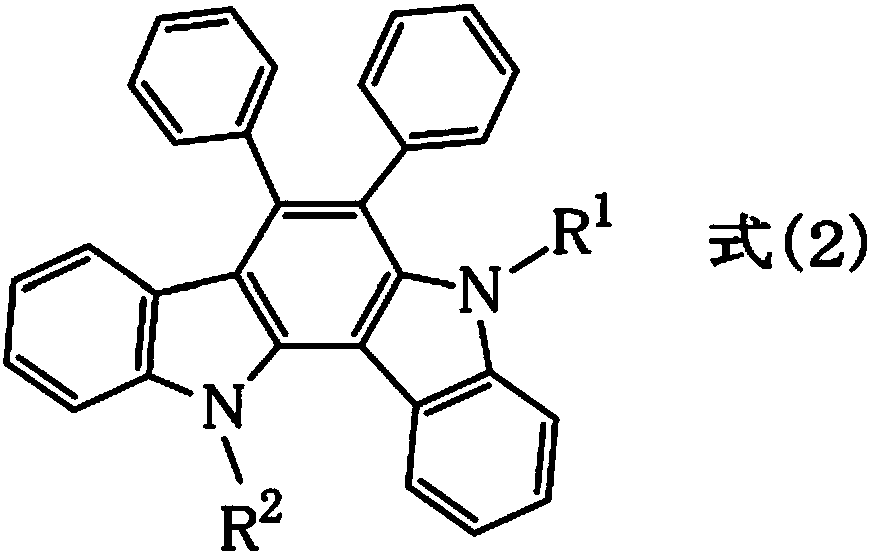
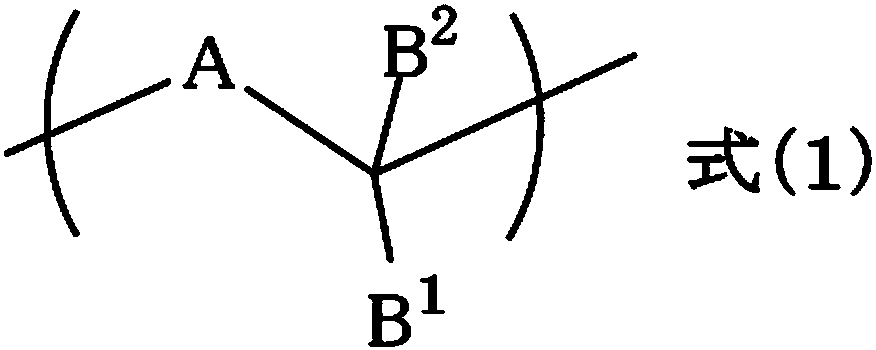
Resist underlayer film forming composition containing indolocarbazole novolak resin
A technology of resist lower layer and composition, which is applied in the direction of coating, photographic plate-making process of pattern surface, photosensitive material used in optical mechanical equipment, etc., can solve the problem of large influence of standing wave of active light and other problems, and achieve Effects of high heat resistance, low amount of sublimation, and high dry etching resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0108]30.00 g of benzil (manufactured by Tokyo Chemical Industry Co., Ltd.), 41.79 g of indole (manufactured by Tokyo Chemical Industry Co., Ltd.), and 5.43 g of p-toluenesulfonic acid monohydrate (manufactured by Tokyo Chemical Industry Co., Ltd.) were added to a 1 L three-necked flask. Co., Ltd.), 300 g of toluene (manufactured by Kanto Chemical Co., Ltd.). Then, a reflux tube was installed, and the mixture was stirred under reflux at 160° C. for about 12 hours under a nitrogen atmosphere. After completion of the reaction, the precipitate was recovered by suction filtration, and washed with 1 L of toluene and 1 L of methanol. The precipitate was dried under vacuum at 60°C for about 12 hours.
[0109] In a 1L three-neck flask, add the dried precipitate and 1,4-di Alkanes 500mL. Then, a reflux tube was installed, and the mixture was stirred under reflux at 120° C. for about 1 hour under a nitrogen atmosphere. After the stirring was stopped, it was cooled and recrystallize...
Synthetic example 2
[0114] Add 8.00 g of compound 1, 2.08 g of benzaldehyde (manufactured by Kishida Chemical Co., Ltd.), 28.39 g of propylene glycol monomethyl ether acetate, and 7.10 g of N-methyl-2-pyrrolidone (Kanto Chemical Co., Ltd.), 1.13 g of methanesulfonic acid (Tokyo Chemical Industry Co., Ltd.). It was then heated up to 150°C and stirred at reflux for about 20 hours. After completion of the reaction, it was diluted with 20.49 g of propylene glycol monomethyl ether acetate, and the precipitate was removed by filtration. The recovered filtrate was added dropwise to methanol solution for reprecipitation. The obtained precipitate was suction-filtered, and the filtrate was dried under reduced pressure at 60° C. overnight. Thus, 6.42 g of a gray powdery resin was obtained. The resulting polymer corresponds to formula (1-2). The weight average molecular weight Mw measured by GPC in terms of polystyrene was 16,100, and the polydispersity Mw / Mn was 3.12.
Synthetic example 3
[0116] Add 8.00 g of compound 1, 3.06 g of 1-naphthaldehyde (manufactured by Tokyo Chemical Industry Co., Ltd.), 32.83 g of propylene glycol monomethyl ether acetate, and 8.21 g of N-methyl-2- Pyrrolidone (manufactured by Kanto Chemical Co., Ltd.), and 2.26 g of methanesulfonic acid (manufactured by Tokyo Chemical Industry Co., Ltd.). It was then heated up to 150°C and stirred at reflux for about 20 hours. After completion of the reaction, it was diluted with 19.37 g of propylene glycol monomethyl ether acetate, and the precipitate was removed by filtration. The recovered filtrate was added dropwise to methanol solution for reprecipitation. The obtained precipitate was suction-filtered, and the filtrate was dried under reduced pressure at 60° C. overnight. Thus, 6.58 g of a gray powdery resin was obtained. The resulting polymer corresponds to formula (1-3). The weight average molecular weight Mw measured by GPC in terms of polystyrene was 7,500, and the polydispersity Mw / M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com