A kind of synthetic method of dihydropyrrolo 2-aminoquinoline compound
A technology of aminoquinoline and dihydropyrrole, which is applied in the field of synthesis of dihydropyrrolo-2-aminoquinoline compounds, can solve the problems of harsh reaction conditions, limitations, poor atom economy, etc., and achieve a convenient scope of application , mild conditions, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
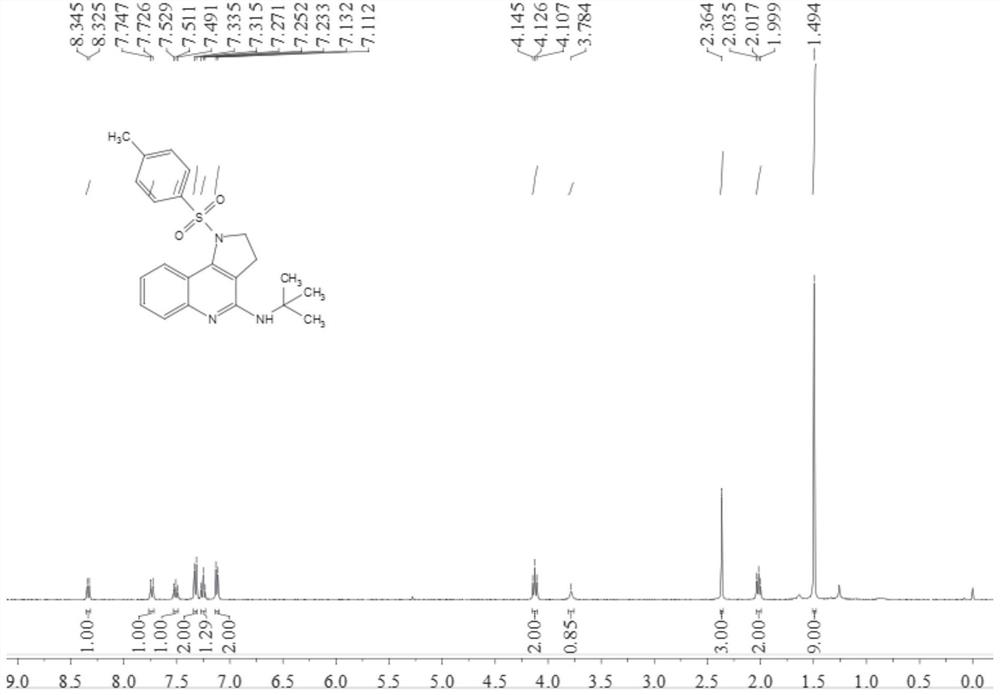
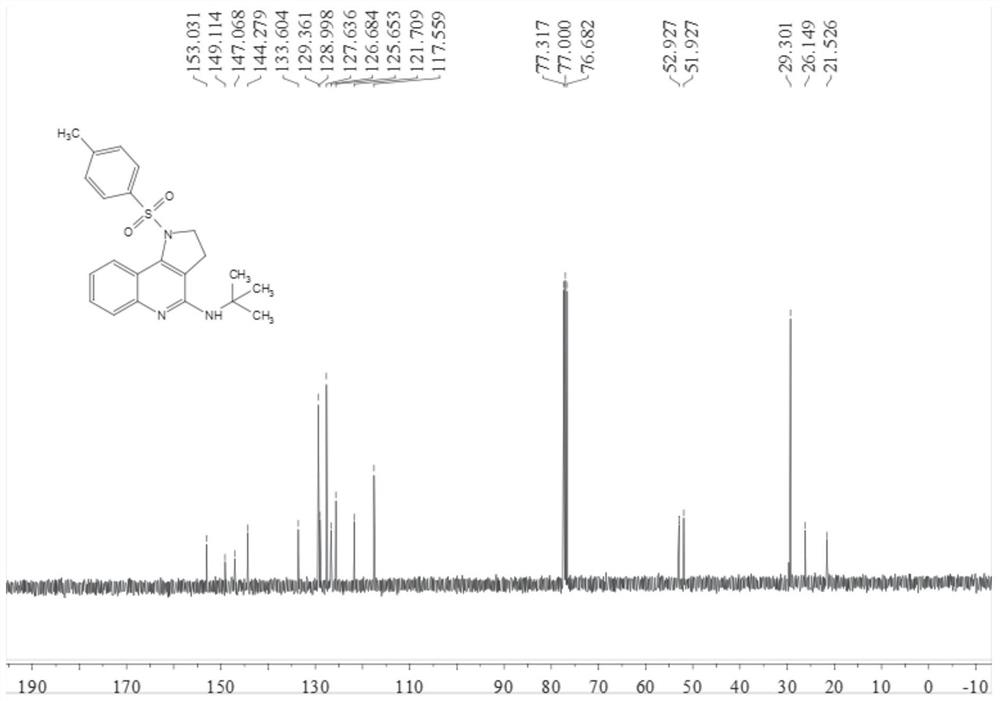
[0038] Add 0.2 mmol N-[4-(2-aminophenyl)but-3-yn-1-yl]-4-methylbenzenesulfonamide, 0.02 mmol tetrakistriphenylphosphine palladium, 0.04 Millimol 1,3-di(diphenylphosphine) propane, 0.4 mmol anhydrous copper acetate and 2 ml of acetonitrile:toluene (1:1, v / v) mixed solvent, finally add 0.3 mmol isonitrile, in Stir and react at 100°C for 9 hours at 700 rpm; stop stirring, add 5 mL of water, extract 3 times with ethyl acetate, combine the organic phases and dry with 0.5 g of anhydrous magnesium sulfate, filter, evaporate the solvent under reduced pressure, and pass through the column Chromatographic separation and purification, the column chromatography eluent used was a mixed solvent of petroleum ether: ethyl acetate with a volume ratio of 10:1 to obtain the target product with a yield of 65%.
[0039] The hydrogen spectrogram and carbon spectrogram of gained target product are respectively as follows figure 1 and figure 2 As shown, the structural characterization data are as ...
Embodiment 2
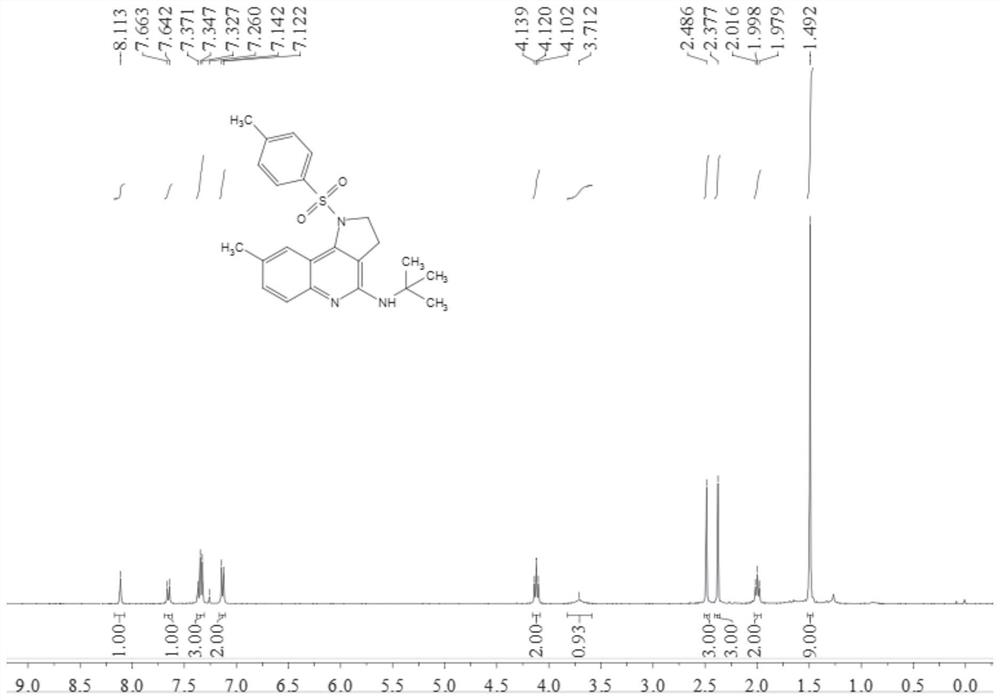
[0047] Add 0.2 mmol N-[4-(2-amino-5-methylphenyl)but-3-yn-1-yl]-4-methylbenzenesulfonamide, 0.02 mmol tetratriphenyl phosphine palladium, 0.04 mmol 1,3-bis(diphenylphosphine) propane, 0.4 mmol anhydrous copper acetate and 2 ml of acetonitrile:toluene (1:1, v / v) mixed solvent, and finally add 0.3 ml mole of tert-butyl isonitrile, stirred and reacted at 100°C for 9 hours at 700 rpm; stopped stirring, added 5 mL of water, extracted 3 times with ethyl acetate, combined the organic phases and dried them with 0.5 g of anhydrous magnesium sulfate, filtered, and evaporated under reduced pressure The solvent was removed, and then separated and purified by column chromatography. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 10:1 to obtain the target product with a yield of 54%.
[0048] The hydrogen spectrogram and carbon spectrogram of gained target product are respectively as follows image 3 and Figure 4 shown; th...
Embodiment 3
[0056] Add 0.2 mmol N-[4-(2-amino-3,5-dimethylphenyl)but-3-yn-1-yl]-4-methylbenzenesulfonamide, 0.02 mmol Four triphenylphosphine palladium, 0.04 mmol 1,3-di(diphenylphosphine) propane, 0.4 mmol anhydrous copper acetate and 2 ml of acetonitrile:toluene (1:1, v / v) mixed solvent, finally Add 0.3 mmoles of tert-butylisonitrile, stir and react at 100°C at 700 rpm for 9 hours; stop stirring, add 5 mL of water, extract 3 times with ethyl acetate, combine organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, The solvent was distilled off under reduced pressure, and then separated and purified by column chromatography. The eluent used in column chromatography was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 10:1 to obtain the target product with a yield of 58%.
[0057] The hydrogen spectrogram and carbon spectrogram of gained target product are respectively as follows Figure 5 and Figure 6 shown; the structural characterization dat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com