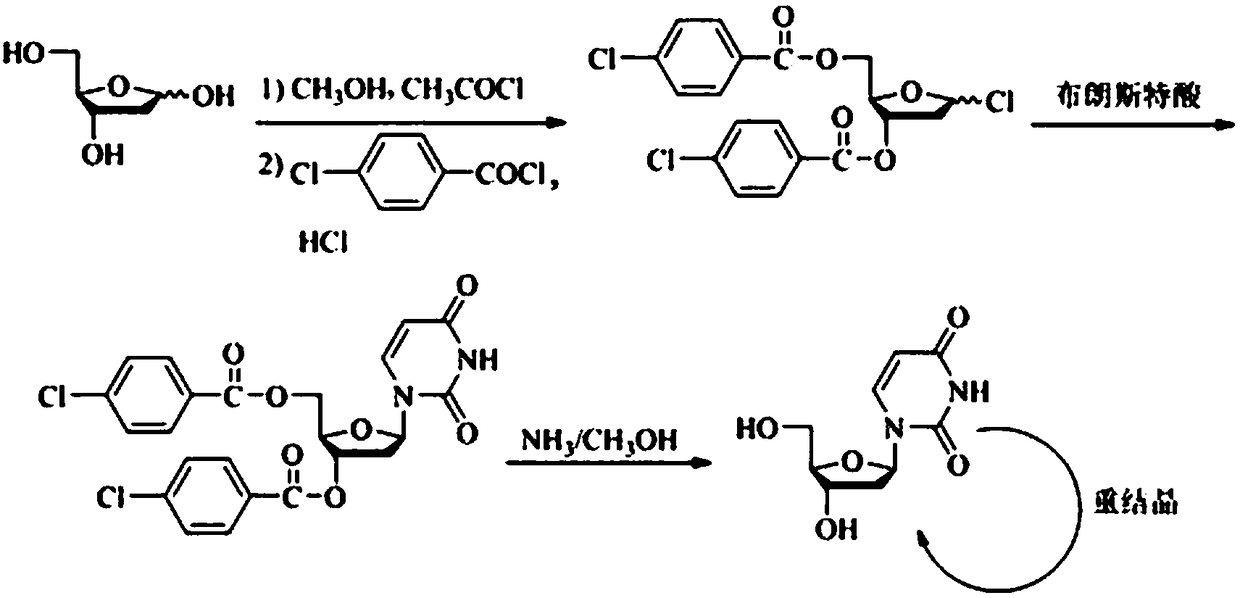
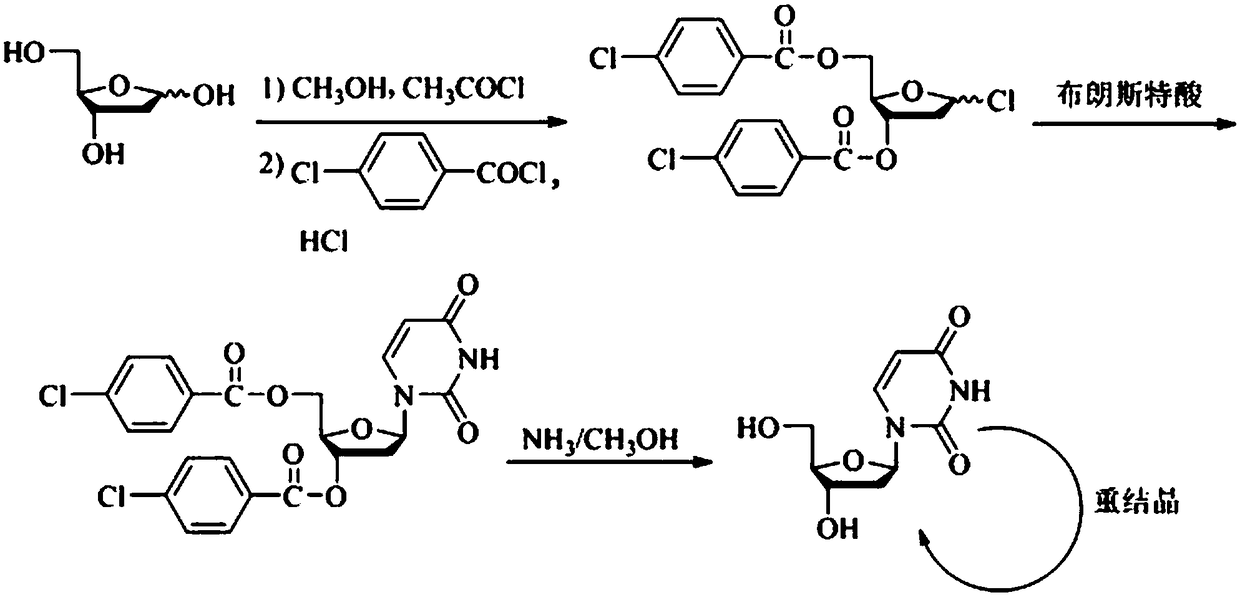
Synthesis method of 2'-deoxy-beta-uridine
A synthesis method and technology of uridine, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems affecting product quality, low selectivity, high impurity content, etc., to improve industrial application prospects, reaction The effect of simple conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Add 2'-deoxyribose (6.00 g, 44.7 mmol) and anhydrous methanol (30 mL) to the reaction flask in sequence, add acetyl chloride (0.17 g, 2.2 mmol) in an ice-water bath and stir for 2 h, then add triethyl The amine (0.33 g, 3.3 mmol) was stirred for 10 min. Distill methanol off under reduced pressure, add 1,4-dioxane (40 mL), DMAP (0.18 mg, 1.5 mmol), triethylamine (9.95 g, 98.3 mmol), add p-chlorobenzyl dropwise under ice-water bath A solution of acid chloride (16.43 g, 93.9 mmol) in 1,4-dioxane (20 mL) was moved to room temperature and stirred for 13 h. Filter, evaporate the solvent under reduced pressure, add 1,4-dioxane (20 mL), acetyl chloride (0.64 g, 8.2 mmol), glacial acetic acid (35 mL), and pass through dry hydrogen chloride gas until TLC shows that the raw material point disappears , filtered, slurried with methyl tert-butyl ether (60 mL×2), and dried to obtain a white solid (14.5 g, yield 75.4%), HPLC purity 89.5%.
Embodiment 2
[0022] Example 2: In the reaction flask, chloroform (30 mL), the white solid obtained in Example 1 (3.0 g, 7.0 mmol), 2,4-bis(trimethylsiloxy)pyrimidine (2.51 g, 9.8 mmol), lithium triflate (0.11 g, 0.7 mmol). Under nitrogen protection, stir for 2 h. Evaporate the solvent under reduced pressure, beat with ethanol (20 mL×2), filter, add methanol (30 mL) to reflux, cool down to crystallize, filter, and dry to obtain a white solid (2.50 g, yield 74.8%), HPLC purity 98.4%.
Embodiment 3
[0023] Example 3: In the reaction flask, chloroform (30 mL), the white solid obtained in Example 1 (3.0 g, 7.0 mmol), 2,4-bis(trimethylsiloxy)pyrimidine (2.51 g, 9.8 mmol), ytterbium triflate (0.44 g, 0.7 mmol). Under nitrogen protection, stir for 2 h. Evaporate the solvent under reduced pressure, beat with ethanol (20 mL×2), filter, add methanol (30 mL) to reflux, cool down to crystallize, filter, and dry to obtain a white solid (2.45 g, yield 73.3%), HPLC purity 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com