Method for quickly detecting heparin disaccharide content in heparin and/or low-molecular heparin
A technology of low molecular weight heparin and heparin disaccharide, applied in the field of drug detection, can solve problems such as long detection time, and achieve the effect of long service life and rapid analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
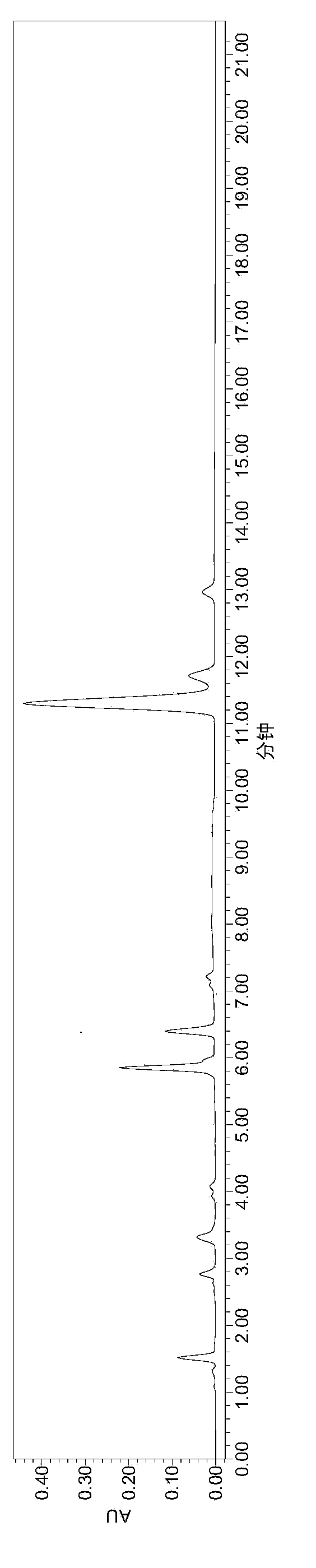
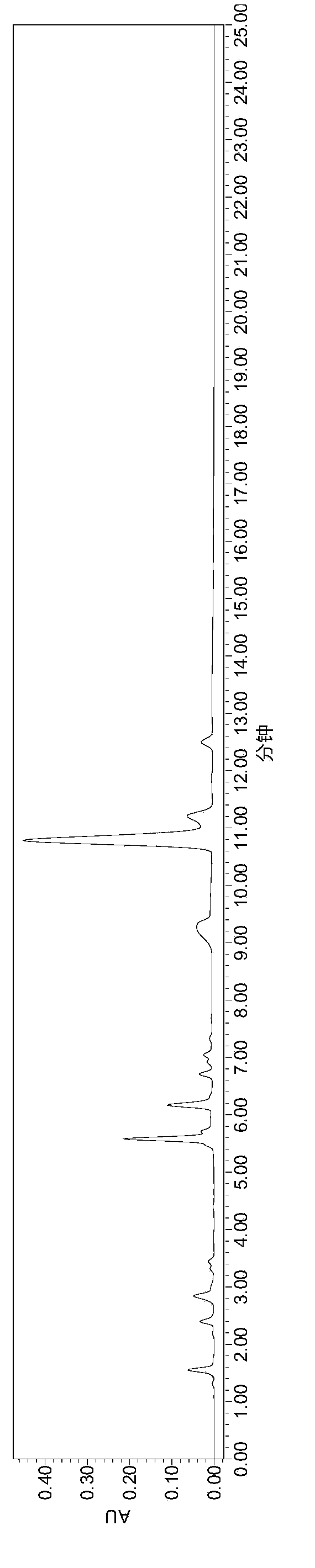
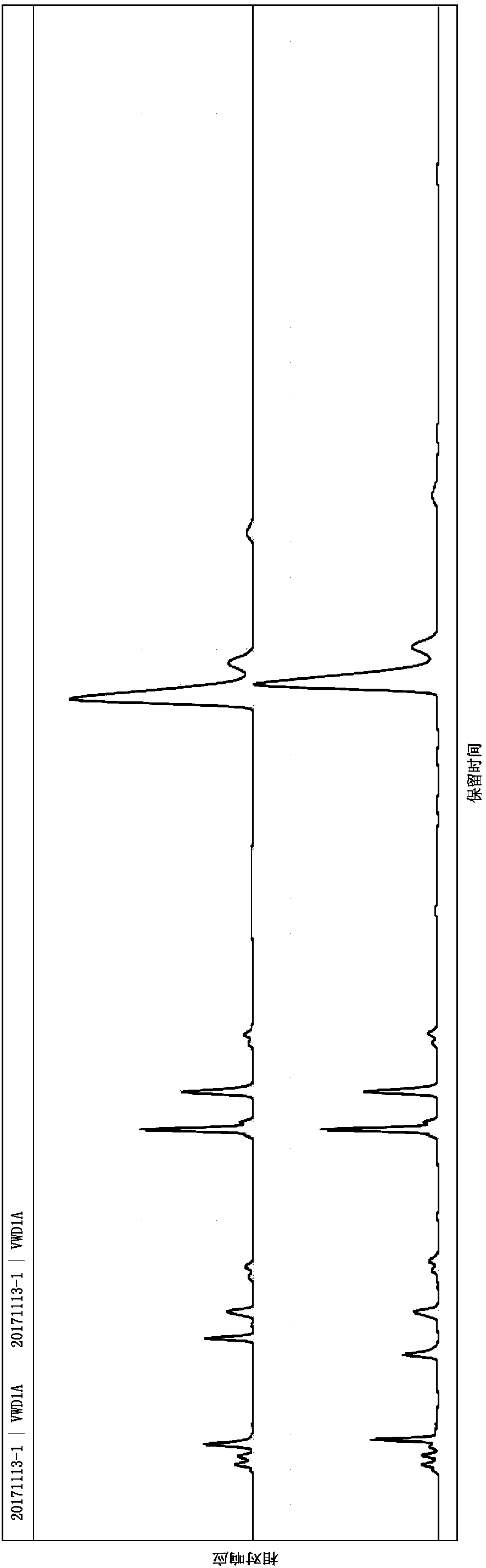
Embodiment 1
[0064] instrument:
[0065] High-performance liquid chromatography (Waters e2695-2489), BSA224S electronic balance (Mettler), low-temperature circulating water tank, FE20pH meter (Mettler).
[0066] Reagent:
[0067] Heparin Sodium (Dongying Tiandong Pharmaceutical Co., Ltd.), Enoxaparin Sodium (Dongying Tiandong Pharmaceutical Co., Ltd.), Heparinase I / II / III (Iduron), Calcium Acetate, Potassium Hydroxide, Sodium Hydroxide, Dihydrogen Phosphate Potassium, bovine serum albumin, purified water, tetrabutylammonium bisulfate, acetonitrile, sodium chloride.
[0068] Preparation of reagents:
[0069] Mobile phase A: prepare 2L of aqueous solution containing 3.3954g tetrabutylammonium hydrogensulfate and 170ml of acetonitrile, that is, 5mmol / L tetrabutylammonium hydrogensulfate and 8.5% aqueous acetonitrile.
[0070] Mobile phase B: prepare 2L of aqueous solution containing 3.3954g tetrabutylammonium hydrogensulfate, 170ml acetonitrile, 35.10g NaCl, that is, 5mmol / L tetrabutylammo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com