Application of compound to treatment of senile dementia through antagonistic protein aggregation
A technology of senile dementia and compound, which is applied in the field of medicine to achieve the effect of improving learning and memory ability and improving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
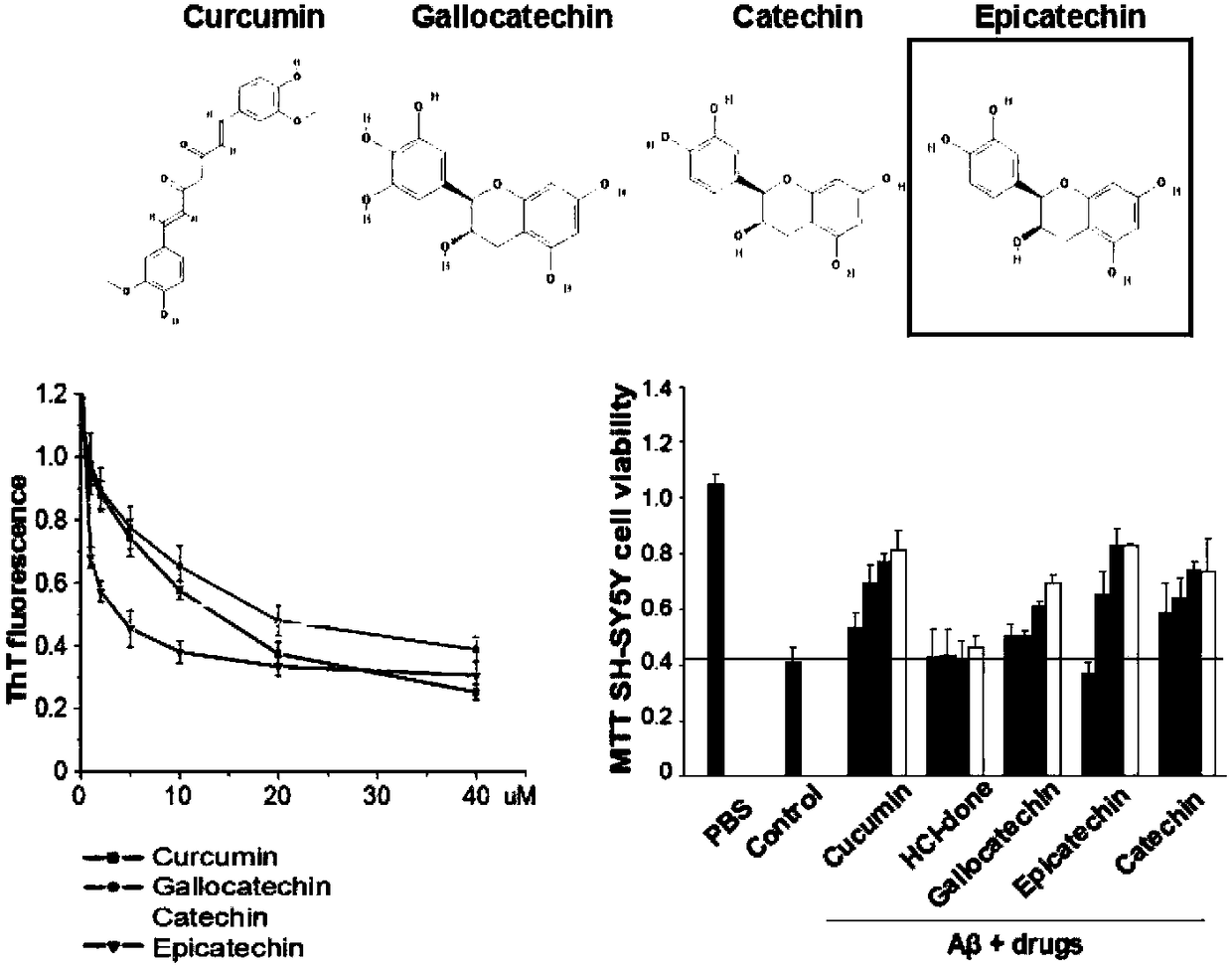
[0028] Example 1 Thioflavin T assay (ThT) for epicatechin inhibiting Aβ42 monomer aggregation and MTT assay for inhibiting Aβ42 oligomer-mediated neurotoxic injury
[0029] The protein Aβ protein was synthesized by Nanjing Taopu Biological Co., Ltd., with a purity greater than 95%. The compound epicatechin was provided by Shanghai Yuanye Biological Company. The Aβ monomer powder is stored at -80°C. When it is taken out for the first time, it should be allowed to stand at room temperature for 30 minutes, then diluted with hexafluoroisopropanol (HFIP) to 1.0 mg / ml, left to stand for at least 2 hours, and ultrasonicated for 30 minutes to destroy the existing polysaccharides. Polymer structure, after drying at low temperature, store at -20°C immediately. Thioflavin T (Thioflavin T) was purchased from Sigma (product number T3516-5g). Weigh 0.032g of ThT powder and dissolve it in 20ml of 20mM Tris-HCl (pH 7.4) solution to obtain 5mM ThT mother solution, which is diluted to 5uM whe...
Embodiment 2
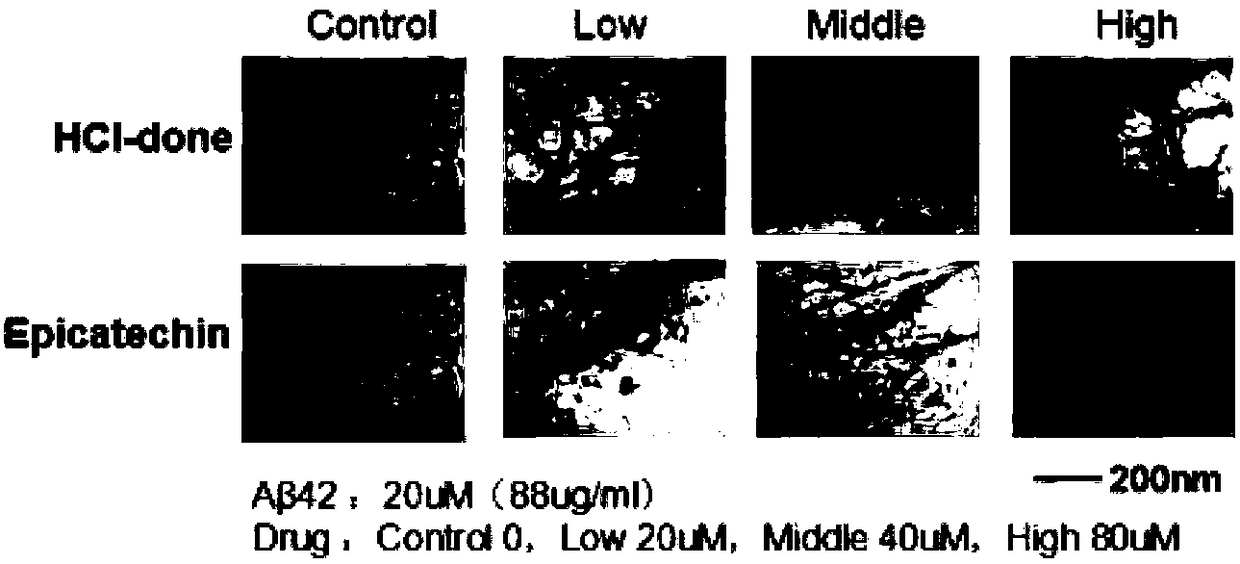
[0034] Example 2 Epicatechin and Aβ Protein Transmission Electron Microscopy Experiment (Transmission electron microscopy, TEM)
[0035] The pretreatment was as in Example 1, and then, 5ul of the Aβ protein sample incubated at 37°C for 24 hours was dropped on the surface of a 300-mesh carbon-coated copper grid, air-dried, and negatively stained with 2% (w / v) sodium phosphotungstic acid After 30 seconds, let stand at room temperature for about 4 hours, use JEM-100CXII transmission electron microscope system (JEOL inc., Tokyo, Japan) to detect and take pictures, and the accelerating voltage is 80kV.
[0036] The observation results are attached figure 2 . Compared with the untreated control group, the fiber density, length and width of the Aβ protein in the experimental group (EPI) added with epicatechin compounds decreased significantly in a concentration-dependent manner, while the other group of cholinesterase inhibitors Aricept (donepezil hydrochloride) has no effect on t...
Embodiment 3
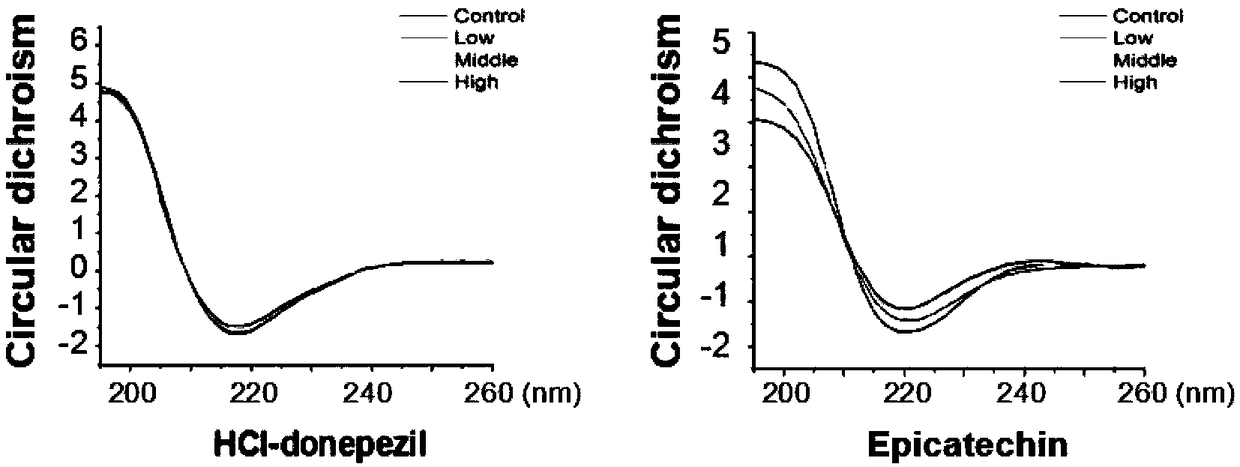
[0037] Example 3 Epicatechin and Aβ protein circular dichroism experiment (CD)
[0038] Aβ monomer was mixed with drugs, the final concentration of Aβ was 20uM, and the final concentrations of drugs were 0uM, 10uM, 20uM and 40uM respectively, and after incubation at 37°C for 12 hours with slow shaking, the J-810 spectrometer (Jasco, Japan) was used to detect. The wavelength range is 190-250nm, the resolution is 0.5nm, the spectral width is 2nm, the scanning speed is 100nm / min, the response time is 1s, and the measurement temperature is normal temperature. The solution was added to a quartz cuvette with an optical path of 0.1 mm for measurement, and each experiment was repeated three times. Subtract the buffer curve from the scan curve obtained each time as the experimental result, each experiment was repeated three times, and the average value was given.
[0039] Using the software Jascow32 data analysis provided by the instrument manufacturer to find (attached image 3 ), t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com