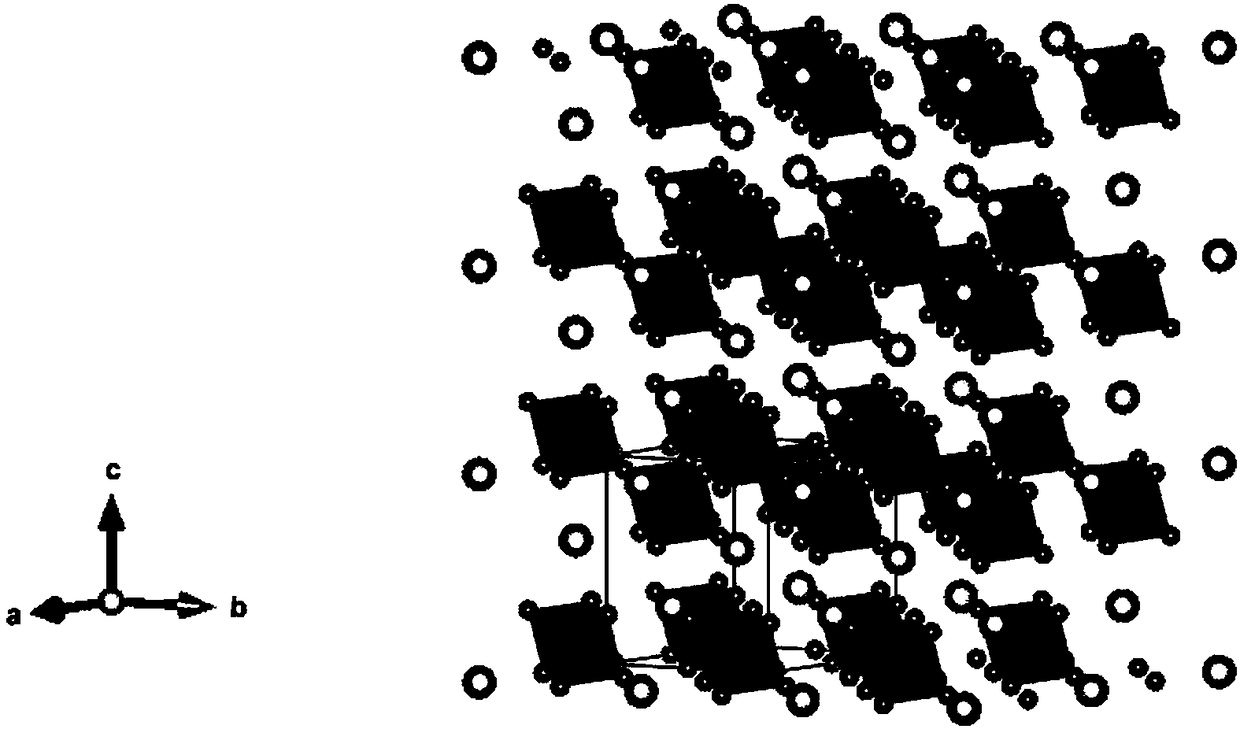
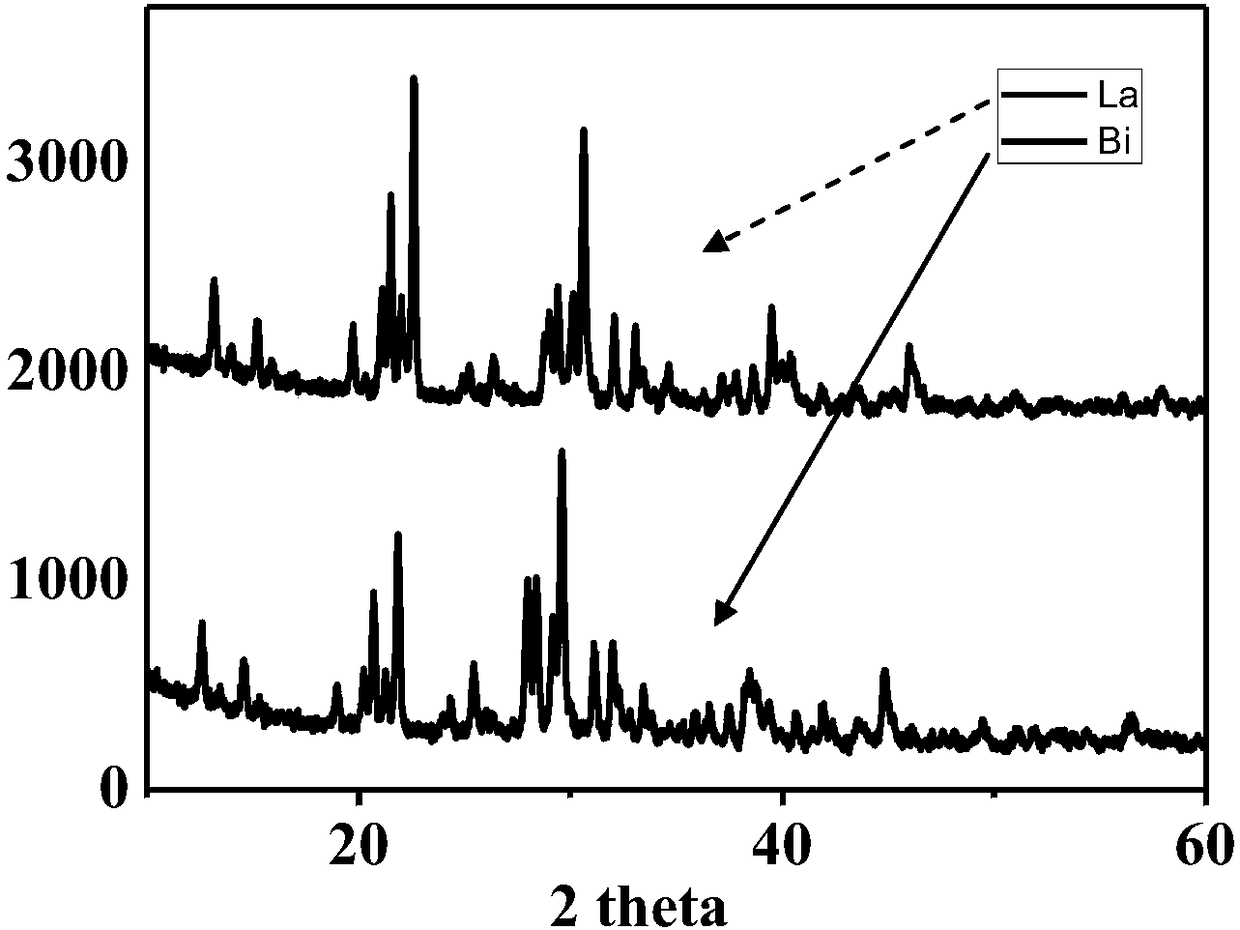
Method for increasing fluorescence yield and stability of non-lead halogen perovskite material
A technology of perovskite material and fluorescence yield, applied in chemical instruments and methods, luminescent materials, etc., can solve problems such as changing luminescence properties, and achieve simple equipment, improved fluorescence quantum yield and stability, and controllable process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A method for improving the fluorescence yield and stability of a lead-free halogen perovskite material, which specifically includes the following steps:
[0050] Step 1: Use acetone, isopropanol, and deionized water to clean the glass bottle and ultrasonically for 15 minutes each, and then dry it with a nitrogen gun;
[0051] Step 2: Methylamine chloride (CH3NH3Cl, 0.336g, 3mmol), antimony chloride (SbCl 3 , 0.456g, 2mmol), erbium chloride hexahydrate (ErCl 3 ·6H 2 (2, 0g, 0mmol) was added to glass vial A. Add 4 mL of hydrochloric acid solution (HCl, 37%) into the glass bottle, and seal the bottle mouth. Obtain the growth precursor;
[0052] Step 3: Put the vessel containing the growth precursor into a heating table or muffle furnace, heat it to 80°C, and keep it at constant temperature for several hours;
[0053] Step 4: Slowly cool the vessel from 80°C to 20°C at a rate of 1°C / hour;
[0054] Step 5: Open the mouth of the container and slowly evaporate the solven...
Embodiment 2
[0058] A method for improving the fluorescence yield and stability of a lead-free halogen perovskite material, which specifically includes the following steps:
[0059] Step 1: Use acetone, isopropanol, and deionized water to clean the glass bottle and ultrasonically for 15 minutes each, and then dry it with a nitrogen gun;
[0060] Step 2: Combine cesium iodide (CsI, 0.504g, 3mmol), indium iodide (InI 3 , 0g, 0mmol), europium oxide (Eu 2 o 3 , 0.352g, 1mmol) into glass vial A. Add 4 mL of hydroiodic acid solution (HI, 37%) into the glass bottle, and seal the bottle mouth. Obtain a single crystal growth precursor;
[0061]Step 3: Put the vessel containing the growth precursor into a heating table or muffle furnace, heat it to 180°C, and keep it at a constant temperature for several hours;
[0062] Step 4: Slowly cool down the vessel from 180°C to 20°C at a rate of 10°C / hour;
[0063] Step 5: Open the mouth of the container and slowly evaporate the solvent in the bottle t...
Embodiment 3
[0067] A method for improving the fluorescence yield and stability of a lead-free halogen perovskite material, which specifically includes the following steps:
[0068] Step 1: Use acetone, isopropanol, and deionized water to clean the container of the hydrothermal kettle and sonicate for 15 minutes each, and then blow dry with a nitrogen gun;
[0069] Step 2: Combine cesium bromide (CsBr, 0.636g, 3mmol), bismuth bromide (BiBr 3 , 0.189g, 1mmol), terbium bromide (TbBr 3 , 0.398g, 1mmol) into the hydrothermal kettle A. Add 4 mL of hydrobromic acid solution (HBr, 37%) into the hydrothermal kettle, and seal the hydrothermal kettle. Obtain the growth precursor;
[0070] Step 3: Put the vessel containing the growth precursor into a heating table or muffle furnace, heat to 120°C, and keep at constant temperature for 10 hours;
[0071] Step 4: Slowly cool the vessel from 120°C to 20°C at a rate of 2°C / hour;
[0072] Step 5: Open the vessel and slowly volatilize the solvent in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com