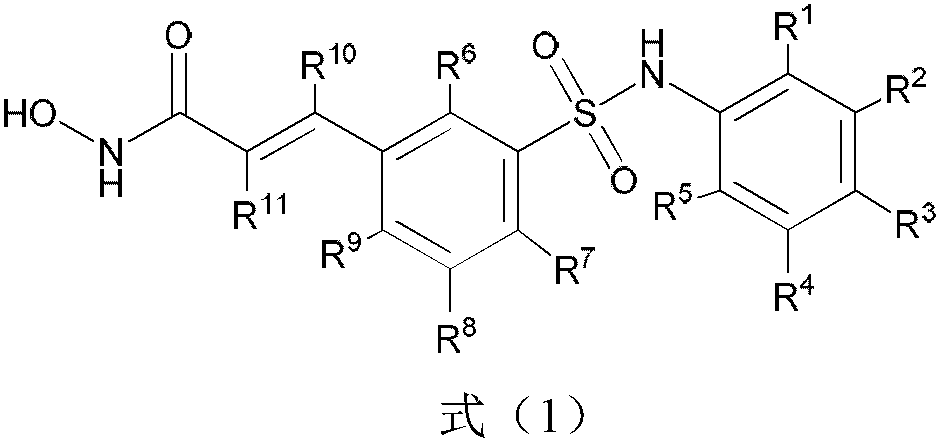
A substituted acrylamide compound and its pharmaceutical composition
A compound and composition technology, applied in the field of medicine, can solve problems such as abnormalities in the blood system and prolongation of the QT interval
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
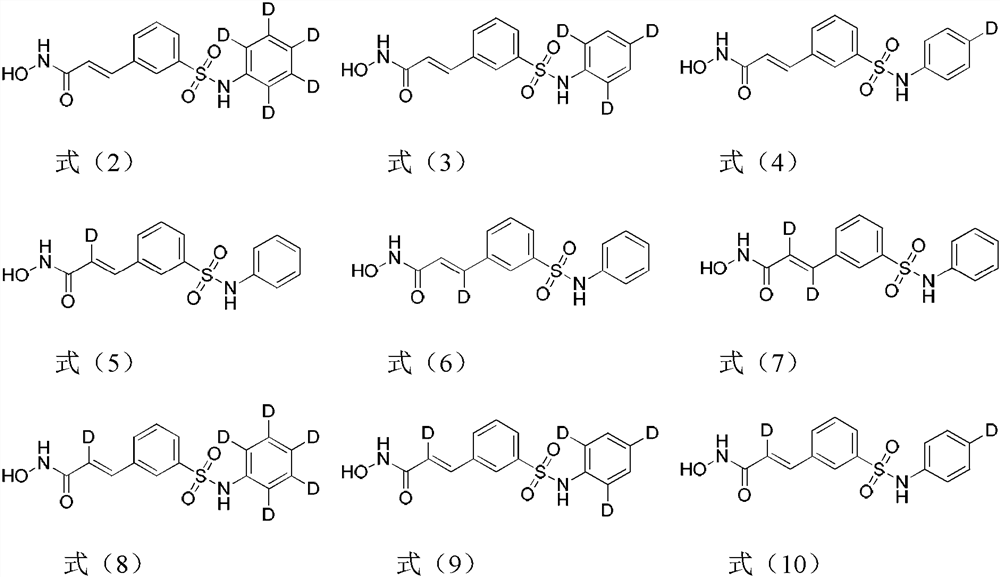
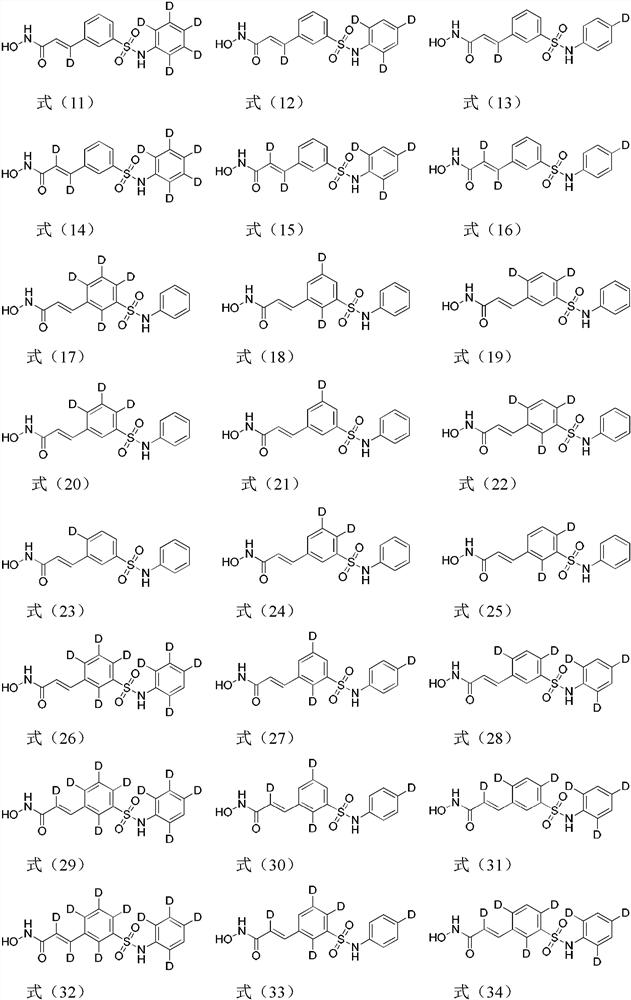
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of N-hydroxyl-3-(3-d5-phenylsulfamoyl-phenyl)-acrylamide (compound 8), using The following route is synthesized:
[0051]
[0052] Step 1 Synthesis of compound 3.
[0053] Dissolve 3-chlorosulfonylbenzoic acid (compound 1, 300 mg) and d7-aniline (compound 2, 0.30 mL) in 10 mL of anhydrous dichloromethane, and stir overnight at room temperature under nitrogen protection. After filtration, the filter cake was washed three times with 10 mL of ice-cold dichloromethane, and the filter cake was collected and dried in vacuo overnight to obtain compound 3 (377 mg) as a white solid product. LC-MS: 281.1 [M-1] - .
[0054] Step 2 Synthesis of compound 4.
[0055] Under the protection of nitrogen, take compound 3 (379 mg) and dissolve it in 10 mL of anhydrous tetrahydrofuran, slowly add BH 3 ·THF (1M, 5.4mL), react overnight at room temperature after the dropwise addition. Add 2 mL of methanol dropwise under cooling in an ice-water bath, stir at ro...
Embodiment 2
[0062] Example 2 Preparation of N-hydroxyl-3-(3-(2,3,5-d3-phenyl)sulfamoyl-phenyl)-acrylamide (compound 15), adopt the following route to synthesize:
[0063]
[0064] Step 1 Synthesis of Compound 10.
[0065] Take aniline (1.023g) and add it to 9mL deuterium water to disperse evenly, add deuterated concentrated hydrochloric acid (1.1mL) to seal, and microwave the reaction at 180°C for 2 hours. Close the microwave reaction device, cool down to room temperature naturally, add solid sodium bicarbonate to adjust the pH to about 8-9, extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, filter and concentrate to obtain a light yellow oily liquid product compound 10 (876 mg). LC-MS: 96.15[M+1] + ; 1 H NMR (500MHz, DMSO-d 6 ) δ7.46 (s, 1H).
[0066] Step 2 Synthesis of Compound 11.
[0067] 3-Chlorosulfonylbenzoic acid (compound 1, 300 mg) and compound 10 (0.30 mL) were dissolved in 10 mL of anhydrous dichloromethane...
Embodiment 3
[0076] Example 3 Preparation of N-hydroxyl-3-(3-phenylsulfamoyl-phenyl)-2-d-acrylamide (compound 21), using The following routes are synthesized:
[0077]
[0078] Step 1 Synthesis of compound 16.
[0079] Take trimethylphosphonoacetate (compound 6, 1.54g) and disperse it in 6mL of deuterium water, add 15mg of potassium carbonate to seal, and react in microwave for 30 minutes at 80 degrees Celsius, close the microwave reactor, cool down to room temperature naturally, ethyl acetate After extraction (10 mL×2), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to give compound 16 (1.471 g) as a colorless oily liquid product. 1 H NMR (300MHz, CDCl 3 ) δ 3.82(s, 3H), 3.78(s, 3H), 3.74(s, 3H).
[0080] Step 2 Synthesis of Compound 17.
[0081] 3-Chlorosulfonylbenzoic acid (compound 1, 300 mg) and aniline (0.30 mL) were dissolved in 10 mL of anhydrous dichloromethane, and stirred overnight at room temperature u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com