Nitrogen heterocyclic compound, application thereof and OLED (organic light-emitting device)
A nitrogen heterocyclic compound and compound technology, applied in the field of organic electroluminescent materials, can solve the problems of unbalanced charge in the light-emitting layer, reduced device efficiency, difficult electron flow, etc., and achieve high luminous purity, good thermal stability, and high luminescence. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
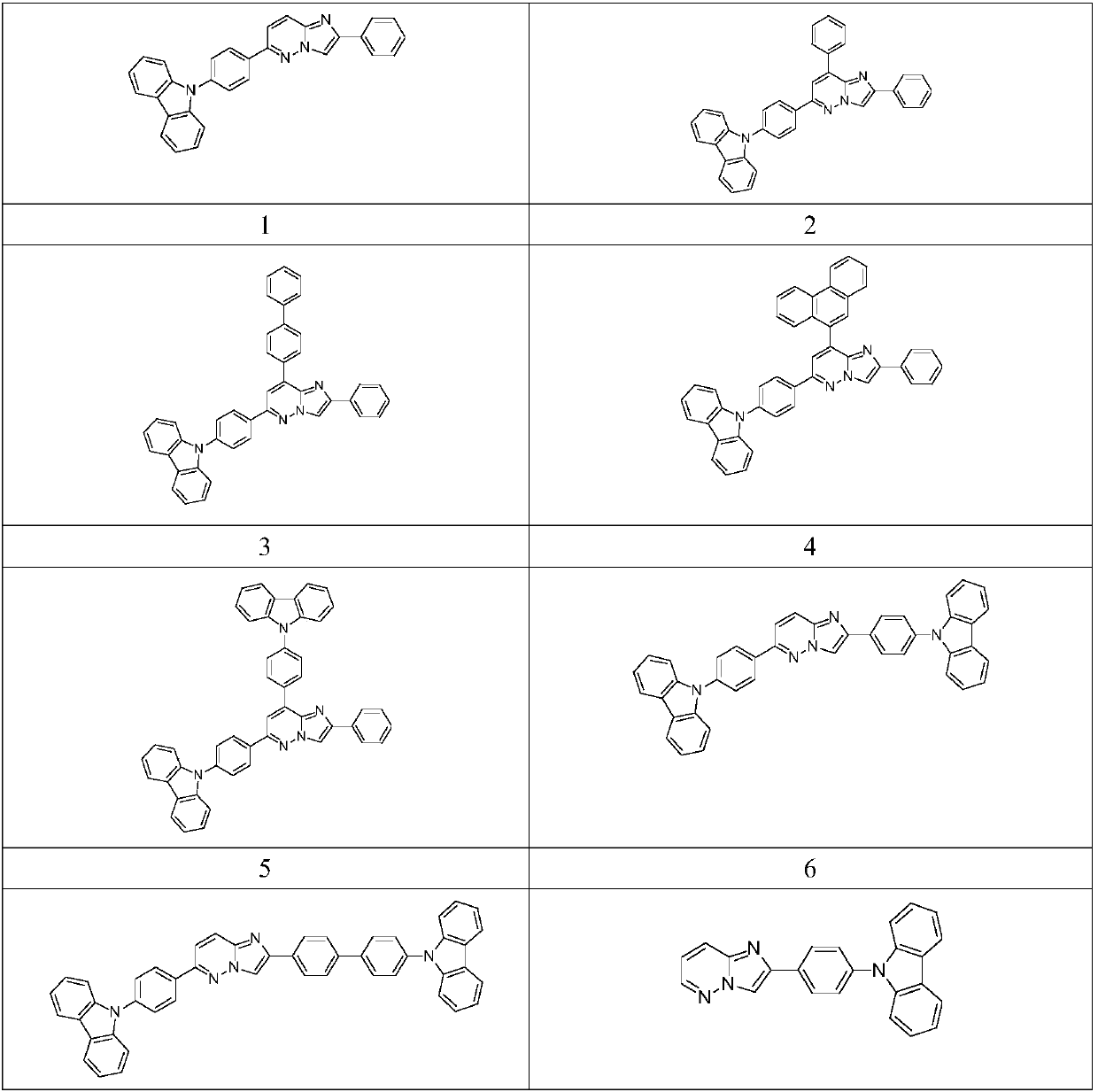
[0049] Synthetic route of compound 1
[0050]
[0051] The synthetic method of intermediate 1-1
[0052] In the flask, add 2-bromoacetophenone (10g, 50mmol), 3-amino-6-chloropyridazine (6.5g, 50mmol), ethanol (80mL), heat and reflux for 6 hours, cool, and filter to obtain the product 7.7 g, yield 67%.
[0053] The synthetic method of compound 1
[0054] In the flask, add intermediate 1-1 (2g, 8.8mmol), 4-(9-carbazolyl) phenylboronic acid (2.5g, 8.8mmol), tetrakistriphenylphosphine palladium (0.1g), potassium carbonate ( 2.5g, 18mmol), tetrahydrofuran (20mL), water (10mL), heated to reflux under nitrogen protection for 10 hours, cooled, extracted with dichloromethane, dried, concentrated, and purified by column chromatography to obtain 3.3g of product, yield 86 %.
Embodiment 2
[0056] Synthetic route of compound 3
[0057]
[0058] The synthetic method of intermediate 3-1
[0059] In the flask, add 2-bromoacetophenone (10g, 50mmol), 3-amino-4-bromo-6-chloropyridazine (10.4g, 50mmol), add ethanol (100mL), heat and reflux for 5 hours, cool , and filtered to obtain product 14g, yield 92%.
[0060] The synthetic method of intermediate 3-2
[0061] In a flask, add Intermediate 3-1 (8g, 26mmol), 4-biphenylboronic acid (5.2g, 26mmol), potassium carbonate (7.2g, 52mmol), tetrahydrofuran (100mL), water (50mL), tetrakistriphenyl Palladium phosphine (0.3g), heated to reflux for 6 hours under the protection of nitrogen, cooled, filtered, and the filter cake was recrystallized with ethanol and tetrahydrofuran to obtain 5.7g of the product with a yield of 58%.
[0062] The synthetic method of compound 3
[0063] The synthesis method was the same as that of compound 1 except that intermediate 1-1 was replaced by intermediate 3-2, and the yield was 82%.
Embodiment 3
[0065] Synthetic route of compound 5
[0066]
[0067] The synthetic method of compound 5
[0068] The synthesis method of compound 1 is consistent with that of compound 1, the raw materials used are intermediate 3-1 and 4-(9-carbazolyl)phenylboronic acid, and the yield is 65%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com