Method for preparing 3-alkynyl isocoumarins compounds
A technology of isocoumarin and compounds, which is applied in the field of preparation of 3-alkynyl isocoumarin compounds, can solve problems such as poor substrate adaptability, poor step economy, severe reaction conditions, etc., and achieve great use value and social Economic benefits, simple steps, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of 3-(3-(tert-butyldimethylsilyloxy)-1-propynyl)-1H-isochromen-1-one (1a)
[0032]
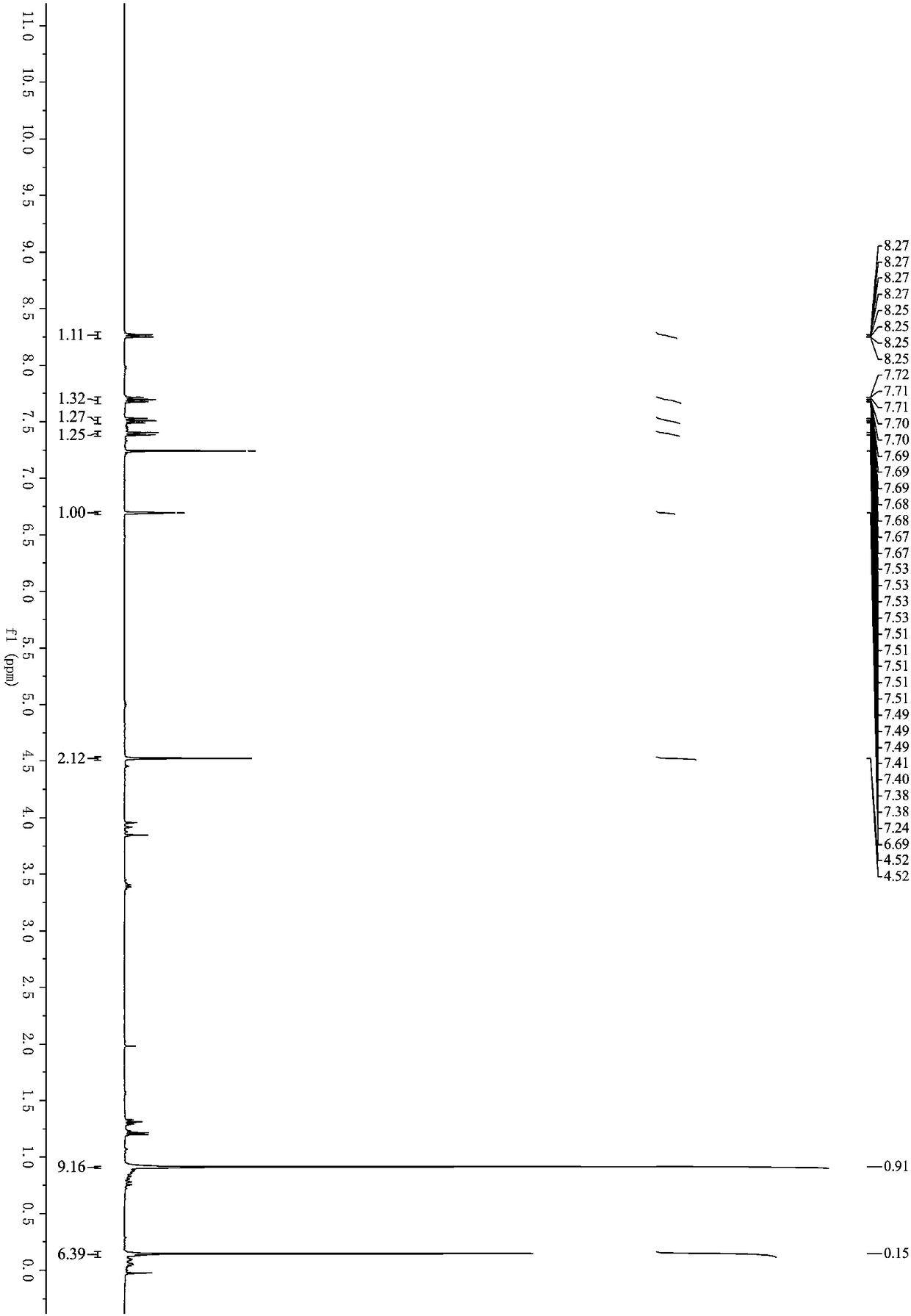
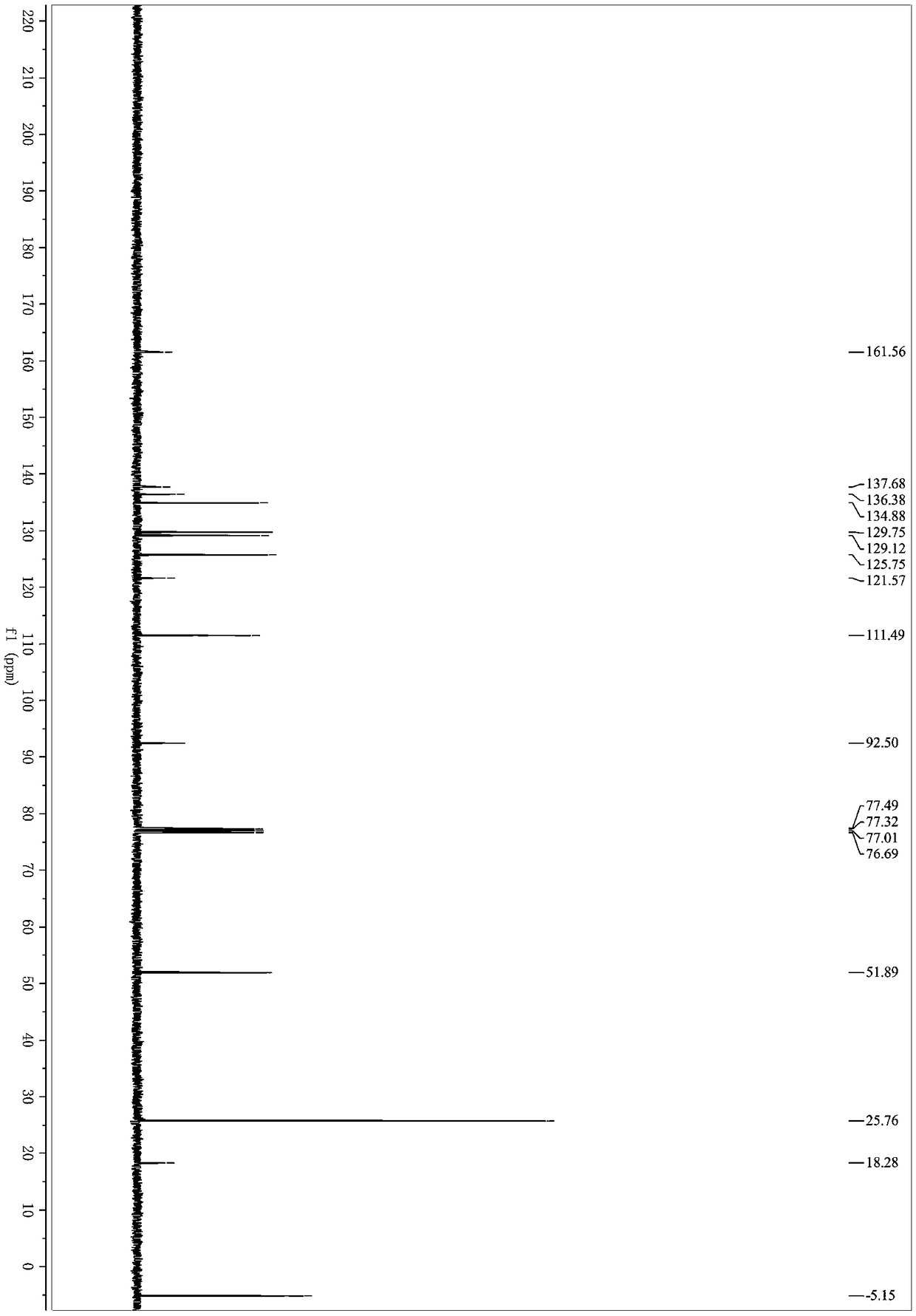
[0033] Under nitrogen protection, methyl 2-(2,2-dibromovinyl)benzoate (318mg, 1mmol), (2-propynyloxy)tert-butyldimethylsilane (170mg, 1mmol), Palladium acetate (11mg, 0.05mmol), cuprous iodide (9mg, 0.05mmol), triphenylphosphine (78mg, 0.3mmol) and cesium carbonate (977mg, 3mmol) were successively added to a 25mL Schlenk bottle, and then added Refined tetrahydrofuran (3mL) was placed in an oil bath for reaction, the reaction temperature was controlled at 70°C, and the reaction was carried out for 4 hours. After the reaction, the organic solvent was removed under reduced pressure; eluting with petroleum ether / ethyl acetate, and separating on a silica gel column, The yield of 3-(3-(tert-butyldimethylsilyloxy)-1-propynyl)-1H-isochromen-1-one was 75%. 1 H NMR (400MHz, Chloroform-d) δ8.26 (ddd, J = 8.0, 1.4, 0.7Hz, 1H), 7.76–7.64(m, 1H), 7.57–7.47(m, 1H), 7.43–7.34(m...
Embodiment 2
[0034] Example 2: Synthesis of 3-(4-(tert-butyldimethylsilyloxy)-1-butynyl)-1H-isochromen-1-one (1b)
[0035]
[0036] Under nitrogen protection, methyl 2-(2,2-dibromovinyl)benzoate (318mg, 1mmol), (3-butynyloxy)tert-butyldimethylsilane (184mg, 1mmol), Palladium acetate (11mg, 0.05mmol), cuprous iodide (9mg, 0.05mmol), triphenylphosphine (78mg, 0.3mmol) and cesium carbonate (977mg, 3mmol) were successively added to a 25mL Schlenk bottle, and then added Refined tetrahydrofuran (3mL) was placed in an oil bath for reaction, the reaction temperature was controlled at 70°C, and the reaction was carried out for 4 hours. After the reaction, the organic solvent was removed under reduced pressure; eluting with petroleum ether / ethyl acetate, and separating on a silica gel column, The yield of 3-(4-(tert-butyldimethylsilyloxy)-1-butynyl)-1H-isochromen-1-one was 72%. 1H NMR (400MHz, Chloroform-d) δ8.24(d, J=7.9Hz, 1H), 7.67(s, 1H), 7.48(t, J=7.7Hz, 1H), 7.36(d, J=7.9Hz ,1H),6.61(s,1H...
Embodiment 3
[0037] Example 3: Synthesis of 3-(4-phenyl-1-butynyl)-1H-isochromen-1-one (1c)
[0038]
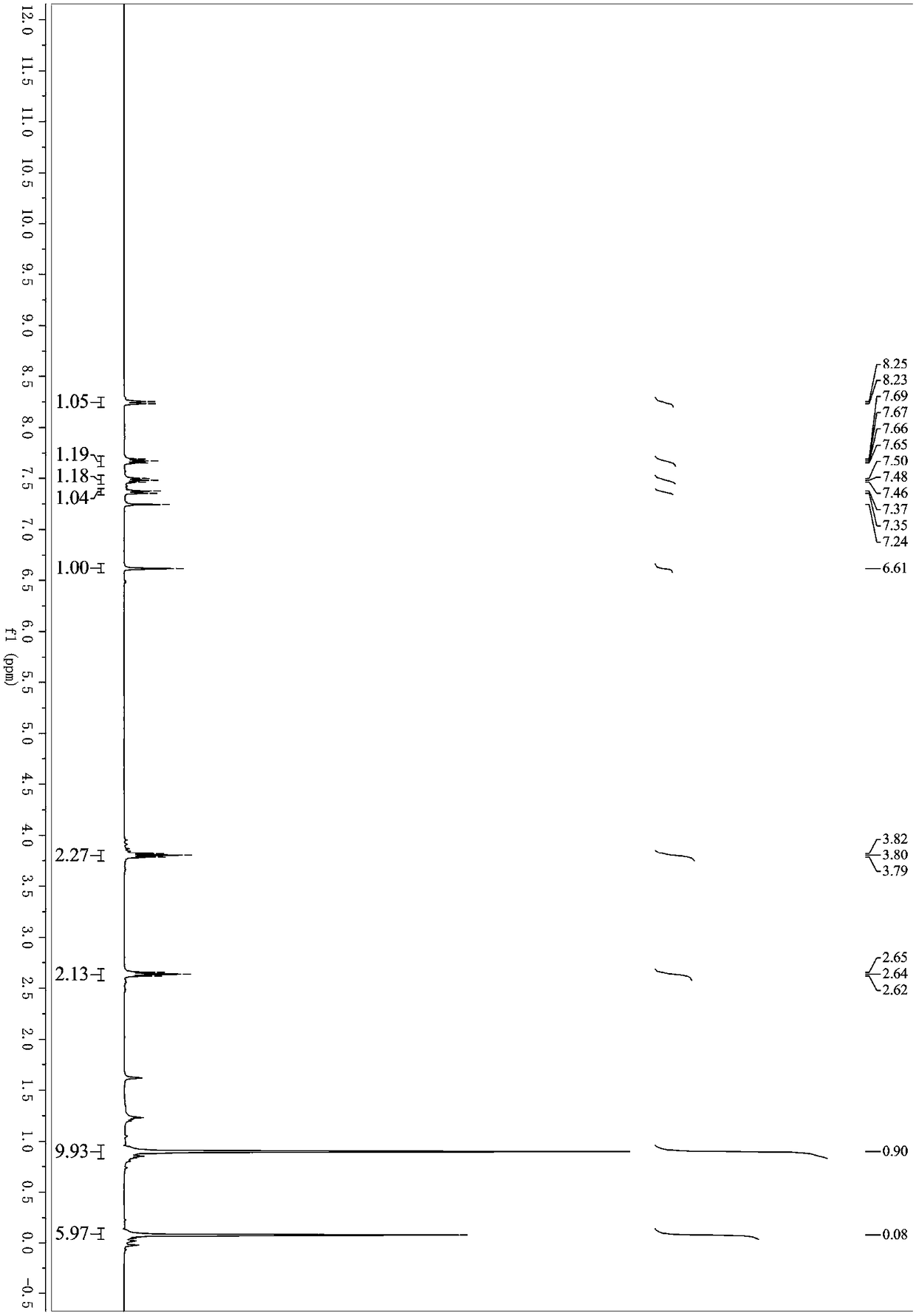
[0039] Under nitrogen protection, methyl 2-(2,2-dibromovinyl)benzoate (318mg, 1mmol), 3-butynylbenzene (130mg, 1mmol), palladium acetate (11mg, 0.05mmol), Cuprous iodide (9mg, 0.05mmol), triphenylphosphine (78mg, 0.3mmol) and cesium carbonate (977mg, 3mmol) were successively added to a 25mL Schlenk bottle, and then purified tetrahydrofuran (3mL) was added and placed React in an oil bath, the reaction temperature is controlled at 70°C, and react for 4 hours. After the reaction, remove the organic solvent under reduced pressure; elute with petroleum ether / ethyl acetate, separate on a silica gel column, and 3-(4-phenyl-1 The yield of -butynyl)-1H-isochromen-1-one was 70%. 1 H NMR (400MHz, Chloroform-d) δ8.25 (d, J = 7.9Hz, 1H), 7.67 (td, J = 7.6, 1.4Hz, 1H), 7.48 (td, J = 7.6, 1.2Hz, 1H) ,7.38–7.28(m,3H),7.27–7.22(m,3H),6.59(s,1H),2.92(t,J=7.5Hz,2H),2.72(t,J=7.5Hz,2H). 13 C NMR (101MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com