Magnetic organic solid base catalyst as well as preparation method and application thereof
A technology of organic solid and alkali catalysts, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as non-reusable, prolong service life, Easy to separate and maintain high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
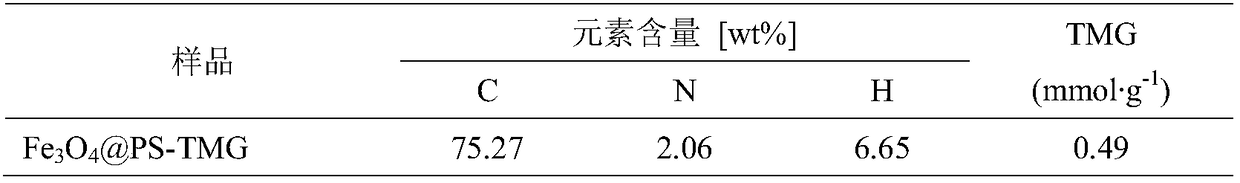
[0045]Add 7.95g of ferrous chloride tetrahydrate, 16.16g of ferric nitrate nonahydrate and 144mL of water into the reactor, mechanically stir to dissolve, pass nitrogen gas for 30min, then add 30mL of ammonia water and 3.4g of oleic acid dropwise, and react at 80°C for 4h. Magnetic separation of Fe 3 o 4 , and washed to neutral with deionized water to obtain Fe 3 o 4 The magnetic core, mixed with n-octane and dried over anhydrous sodium sulfate, gave Fe 3 o 4 ferrofluid.
[0046] Weigh 7.5g of magnetic fluid, 6.83g of styrene, 0.68g of divinylbenzene, 3.3g of p-chloromethylstyrene, and 0.285g of n-hexadecane to form an oil phase, and ultrasonically disperse for 10 minutes. Weigh 1.35g of sodium lauryl sulfate and dissolve it in 135mL of deionized water to obtain a water phase. Under mechanical stirring, add the oil phase into the water phase drop by drop, and stir for 2 hours to obtain a black oil drop suspension. Micro-emulsify in an ice bath for 10 min to obtain a unif...
Embodiment 2
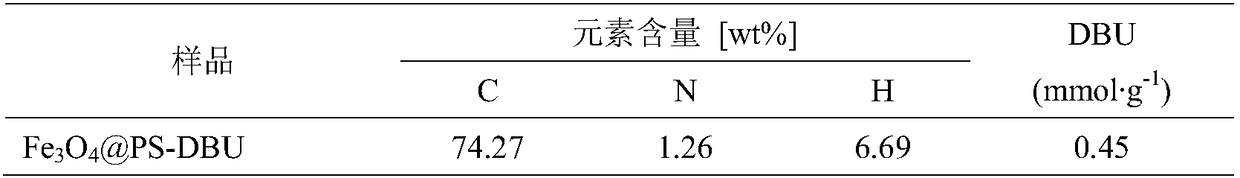
[0050] Add 7.95g of ferrous chloride tetrahydrate, 16.16g of ferric nitrate nonahydrate and 144mL of water into the reactor, mechanically stir to dissolve, pass nitrogen gas for 30min, then add 30mL of ammonia water and 3.4g of oleic acid dropwise, and react at 80°C for 4h. Magnetic separation of Fe 3 o 4 , and washed to neutral with deionized water to obtain Fe 3 o 4 The magnetic core, mixed with n-octane and dried over anhydrous sodium sulfate, gave Fe 3 o 4 ferrofluid.
[0051] Weigh 7.5g of magnetic fluid, 3.4g of styrene, 0.76g of divinylbenzene, 3.3g of p-chloromethylstyrene, and 0.57g of n-hexadecane to form an oil phase, and ultrasonically disperse for 10 minutes. Weigh 1.35g of sodium lauryl sulfate and dissolve it in 135mL of deionized water to obtain a water phase. Under mechanical stirring, add the oil phase into the water phase drop by drop, and stir for 2 hours to obtain a black oil drop suspension. Micro-emulsify in an ice bath for 10 min to obtain a unifo...
Embodiment 3
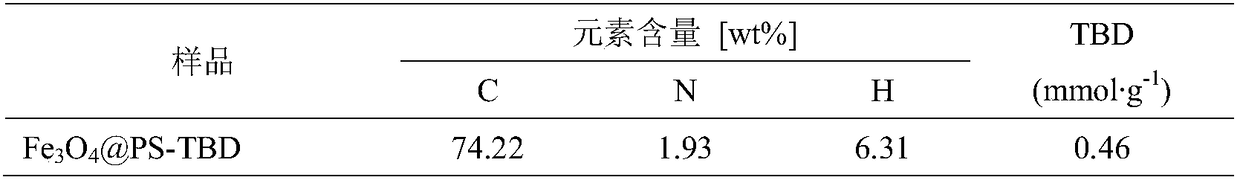
[0055] Add 5.07g of ferrous chloride, 6.49g of ferric chloride and 144mL of water into the reactor, mechanically stir to dissolve, pass nitrogen gas for 30min, then add 30mL of ammonia water and 3.4g of oleic acid dropwise, and react at 80°C for 4h. Magnetic separation of Fe 3 o 4 , and washed to neutral with deionized water to obtain Fe 3 o 4 The magnetic core, mixed with n-octane and dried over anhydrous sodium sulfate, gave Fe 3 o 4 ferrofluid.
[0056] Weigh 7.5g of magnetic fluid, 6.83g of styrene, 0.68g of divinylbenzene, 3.3g of p-chloromethylstyrene, and 0.285g of n-hexadecane to form an oil phase, and ultrasonically disperse for 10 minutes. Weigh 1.35g of sodium lauryl sulfate and dissolve it in 169mL of deionized water to obtain a water phase. Under mechanical stirring, add the oil phase into the water phase drop by drop, and stir for 2 hours to obtain a black oil drop suspension. Micro-emulsify in an ice bath for 10 min to obtain a uniform and stable micro-emu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com