Perfluoroalkyl sulfamide active amine acrylate compound and preparation method
A technology of active amine acrylate and perfluoroalkyl sulfonamide, which is applied in the preparation of sulfonate amide, organic chemistry, polyurea/polyurethane coating, etc., can solve the problem of difficult to wipe, can not be effectively inhibited or overcome, light-cured coating surface Not resistant to stains and other problems, to achieve the effect of high surface firmness, perfect surface curing, anti-fouling and wipe resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
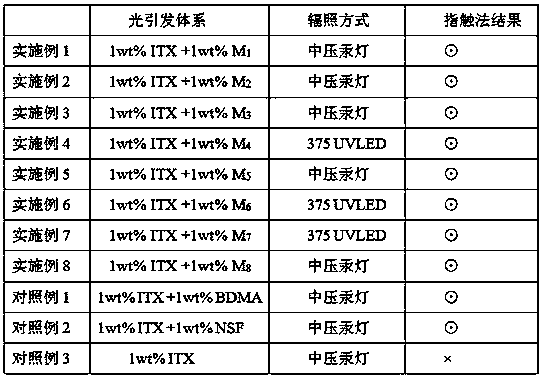
Examples
Embodiment 1
[0044] A perfluoroalkylsulfonamide active amine acrylate compound, its structure is as follows: 1 :
[0045] , Formula M 1 ;
[0046] The above-mentioned perfluoroalkylsulfonamide active amine acrylate compound (M1) is prepared by the following method:
[0047] S1. Take N-aminoethylpiperazine and perfluorooctyl sulfonyl fluoride as raw materials to prepare a fluorocarbon chain compound A1 containing primary or secondary amine sulfonamide groups. The structure of compound A1 is as follows:
[0048] , A 1 ;
[0049] Using tetrahydrofur as the solvent, add 1 mol of perfluorooctyl sulfonyl fluoride dropwise to 3 mol of N-aminoethyl piperazine at 0°C. After the addition is complete, stir for 30 minutes and react at room temperature for 3 hours. A white precipitate is produced. ; Filter, wash with acetone 3 times, and dry to obtain white powdery solid compound A 1 , The yield is 76.13%. After NMR testing, the testing data is: 1 H NMR (400 MHz, DMSO) δ3.08 – 3.02 (1H), 2.82 (2H), 2.77 – 2....
Embodiment 2
[0053] A preparation of perfluoroalkyl sulfonamide active amine acrylate compound (M 1 The method of ), except that the raw material feeding ratio, reaction temperature, and reaction time are different, other conditions are the same as in Example 1;
[0054] Wherein, the method is carried out according to the following steps:
[0055] S1. Using tetrahydrofur as the solvent, add 1 mol of perfluorooctyl sulfonyl fluoride to 1 mol of N-aminoethyl piperazine dropwise at -30°C, and after the addition is complete, stir for 30 minutes and react at room temperature for 5 hours. White precipitate is produced; filtered, washed with acetone 3 times, and dried to obtain white powdery solid compound A 1 .
[0056] S2. Put 1mol of compound A 1 , 1mol 1,6-hexanediol diacrylate was added to the acetonitrile solution, stirred at 50°C for 12 hours, and the solvent was spin-dried to obtain a light brown liquid; after nuclear magnetic testing, the obtained light brown liquid contained the product perflu...
Embodiment 3
[0058] A preparation of perfluoroalkyl sulfonamide active amine acrylate compound (M 1 The method of ), except that the raw material feeding ratio, reaction temperature, and reaction time are different, other conditions are the same as in Example 1;
[0059] Wherein, the method is carried out according to the following steps:
[0060] S1. Using tetrahydrofur as the solvent, add 1 mol of perfluorooctyl sulfonyl fluoride to 10 mol of N-aminoethyl piperazine dropwise at 30°C. After the addition is complete, stir for 30 minutes and react at room temperature for 4 hours, white. Precipitation occurs; filtered, washed with acetone 3 times, and dried to obtain white powdery solid compound A 1 .
[0061] S2. Put 1mol of compound A 1 , 5mol 1,6-hexanediol diacrylate was added to the acetonitrile solution, stirred at 100°C for 24 hours, and the solvent was spin-dried to obtain a light brown liquid; after nuclear magnetic testing, the obtained light brown liquid contained the product perfluoroal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com