A kind of preparation method of 3'-oxygen-methoxyethyl nucleoside
A technology for methoxyethyl nucleoside and methylcytosine, which is applied in the field of nucleoside compound synthesis, can solve the problem of difficulty in large-scale production, no method suitable for industrialized preparation of 3'-oxy-methoxyethyl nucleoside, and low yield And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
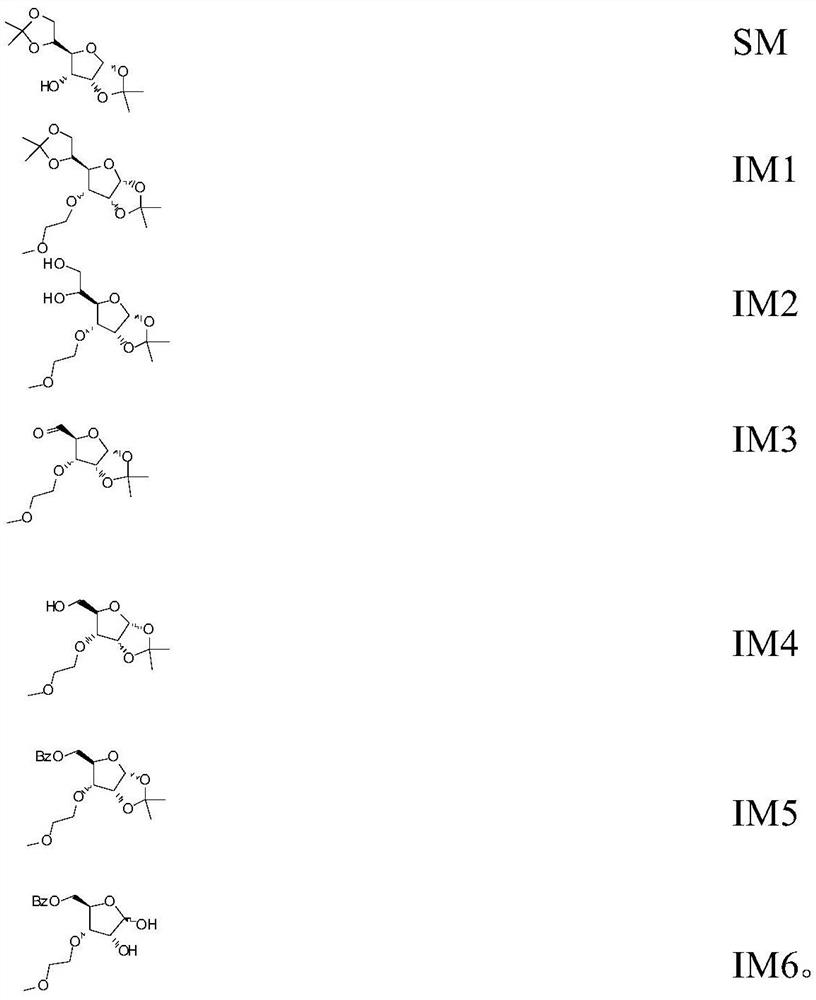
[0094] Preparation of 3'-Oxy-methoxyethyl-5-methyluridine
[0095] 1.1,2; 5,6-Diacetonylidene-3-methoxyethyl allose (IM1) preparation:
[0096]
[0097] Under the protection of argon, weigh 60.0g SM (1,2; 5,6-diacetonylidene allose), add 300mL DMSO to dissolve, stir 300mL 50% KOH aqueous solution for 5 minutes, add 43.6g 2- Chloroethyl methyl ether, heated to 55 ° C reaction. After reacting for 16 hours, sampling TLC detection showed that the raw material SM had all disappeared, and a new point was formed on the upper part of the raw material.
[0098] In the reaction process, the TLC detection condition is: after ethyl acetate / n-hexane=3 / 5 system develops, with the methanol solution of 5% sulfuric acid immerse plate, heat gun blows plate to show black spot after the methanol solution of 5% sulfuric acid, raw material Rf:0.25, product Rf: 0.3.
[0099] After the reaction was completed, 800 mL of dichloromethane was added to the reaction solution for dilution, poured into...
Embodiment 2
[0123] Preparation of 3'-oxo-methoxyethyl-5-methylcytidine (A-2)
[0124]
[0125] Weigh 22.5g of Compound B, suspend 6.5g of 5-methylcytosine in 250mL of acetonitrile, add 26.4g of BSA dropwise, and raise the temperature to 75°C to dissolve the system. After the reaction liquid was lowered to 5°C, 14.4 g of TMSOTf was added dropwise, and the reaction temperature returned to 75°C. After completion of the reaction as monitored by HPLC, the reaction was quenched. The reaction solution was diluted with ethyl acetate, washed with water, and the organic phase was dried and concentrated to obtain crude product C-2. The crude product was dissolved in 200 mL methanol / ammonia water, and stirred at 25°C overnight. After the reaction was completed, it was concentrated, and 100 mL of absolute ethanol was added for crystallization to obtain 10.5 g of the product 3'-oxy-methoxyethyl-5-methylcytidine (A-2), with a purity of 99.9% and a yield of 77.0%.
[0126] 1 H NMR (500MHz, DMSO-d ...
Embodiment 3
[0128] Preparation of 3'-Oxy-methoxyethyl adenosine (A-3)
[0129]
[0130] Weigh 26.0g of compound B, suspend 8.1g of adenine in 250mL of acetonitrile and 1,2-dichloroethane, cool down to 10°C, add 26.1g of tin tetrachloride dropwise, and stir at 25°C to react after the dropwise addition. After completion of the reaction as monitored by HPLC, it was quenched with water. The reaction solution was diluted with dichloromethane, washed with water, and the organic phase was dried and concentrated to obtain crude product C-3. The crude product was dissolved in 200 mL methanol / ammonia water, and stirred at 25°C overnight. After the reaction was completed, it was concentrated and chromatographically obtained 12.1 g of the product 3'-oxygen-methoxyethyladenosine (A-3), with a purity of 99.6% and a yield of 70%.
[0131] 1 H NMR (500MHz, DMSO-d 6 )δ (ppm): 8.35 (s.1H), 8.15 (s, 1H), 7.34 (s.2H), 5.89 (d, J = 8.5Hz, 1H), 5.48-5.45 (m, 1H), 5.41 ( d,J=8.5Hz,1H),4.77-4.73(m,1H),4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com