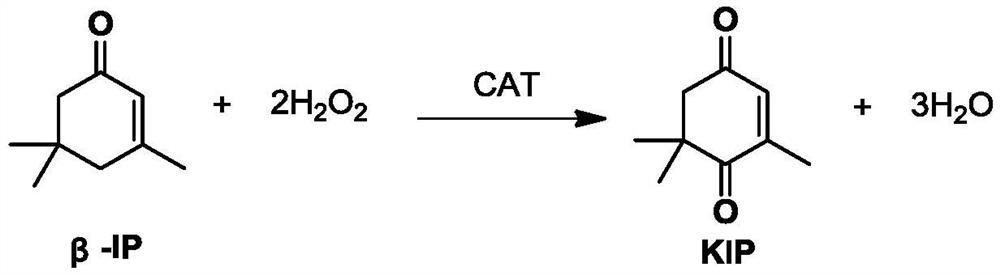
A method for preparing 4-oxoisophorone by solid-liquid two-phase catalytic oxidation of β-isophorone
A technology for oxoisophorone and isophorone is applied in the field of solid-liquid two-phase catalytic oxidation of β-isophorone to prepare 4-oxoisophorone, which can solve the problem of not mentioning 4-oxoisophorone. Isophorone, long time, low conversion rate, etc., to achieve the effect of maintaining stable catalytic activity, mild reaction conditions, and environmental protection of the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of catalyst:
[0059] Take a certain amount of Cu(NO 3 ) 2 ·H 2 O, Al(NO 3 ) 3 9H 2 O, Fe(NO 3 ) 3 9H 2 O, Co(NO 3 ) 3 ·6H 2 O, Mg(NO 3 ) 2 ·6H 2 O, anhydrous Na 2 CO 3 After dissolving in water, prepare 1mol / L standard aqueous solution respectively. Weigh 970mLNa 2 CO 3 Heat the aqueous solution to 70°C in a constant temperature water bath, mix 623mL aluminum nitrate aqueous solution with 19mL copper nitrate, 6.3mL cobalt nitrate, 2.1mL iron nitrate, 2.5mL magnesium nitrate aqueous solution, and slowly add it dropwise to Na 2 CO 3 in solution. During the dropping process, the temperature was controlled at 70±5°C. After the dropping was completed, the constant temperature stirring was continued for 40 minutes, and then the precipitate was poured into a beaker and aged for 24 hours at room temperature. After the precipitate was filtered, the precipitate was washed with deionized until the washing solution was neutral. Then, the filtered ...
Embodiment 2
[0063] Preparation of 4-oxoisophorone:
[0064] The catalyst obtained in Example 1 was washed and air-dried naturally, and used as the catalyst of Example 2.
[0065] A reactor equipped with a six-blade turbine high-speed stirring paddle is used as the reactor. Add 1380g of β-isophorone, 970g of ethanol, 1.38g of sodium carbonate, 13.8g of 1# catalyst, and 13.8g of azobisisobutylimidazoline hydrochloride successively in the reaction kettle; turn on electric heating and mechanical stirring, and react The liquid temperature was raised to 75°C, 2289g of 30% hydrogen peroxide solution was added dropwise for 6 hours, and the temperature was continued for 2 hours. After the reaction, gas chromatography analysis showed that the conversion rate of the raw material β-isophorone was 99.33%. The reaction liquid was filtered, and the catalyst was washed with ethanol, and the catalyst washing liquid was combined with the reaction liquid. After removing the solvent with a rotary evaporato...
Embodiment 3
[0067] Preparation of 4-oxoisophorone:
[0068] The catalyst obtained in Example 2 was washed and air-dried naturally, and used as the catalyst of Example 3.
[0069] A reactor equipped with a six-blade turbine high-speed stirring paddle is used as the reactor. Add 1380g of β-isophorone, 690g of methanol, 3.45g of potassium carbonate, 13.8g of 1# catalyst, and 34.5g of azobisisobutylimidazoline hydrochloride successively in the reaction kettle; turn on electric heating and mechanical stirring, and react The liquid temperature was raised to 60°C, 1387g of 50% hydrogen peroxide solution was added dropwise for 6 hours, and the temperature was continued for 2 hours. After the reaction, gas chromatography analysis showed that the conversion rate of the raw material β-isophorone was 99.25%. The reaction liquid was filtered, and the catalyst was washed with methanol, and the catalyst washing liquid was combined with the reaction liquid. After removing the solvent with a rotary evap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com