Kit for quantitatively detecting D-dimer and preparation method thereof
A quantitative detection and dimer technology, applied in the field of biomedicine, can solve the problems of poor specificity and low sensitivity, and achieve the effect of high specificity, high sensitivity and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of D-dimer acridinium lipid-labeled monoclonal antibody
[0052] 1) Weigh an appropriate amount of D-dimer monoclonal antibody, and use 0.05mol / L pH 9.5 CB to adjust to 1mg / mL;
[0053] 2) Acridine lipid and antibody are calculated according to the molar ratio of 1:15, dissolved in DMF, mixed, and reacted at room temperature for 30 minutes;
[0054] 3) The reaction solution was transferred to a dialysis bag (molecular weight cut-off 8000-12000), and dialyzed with 0.01M PBS pH 7.2 for 24 hours;
[0055] 4) Add an equal volume of glycerin and 0.1% PC-300.
Embodiment 2
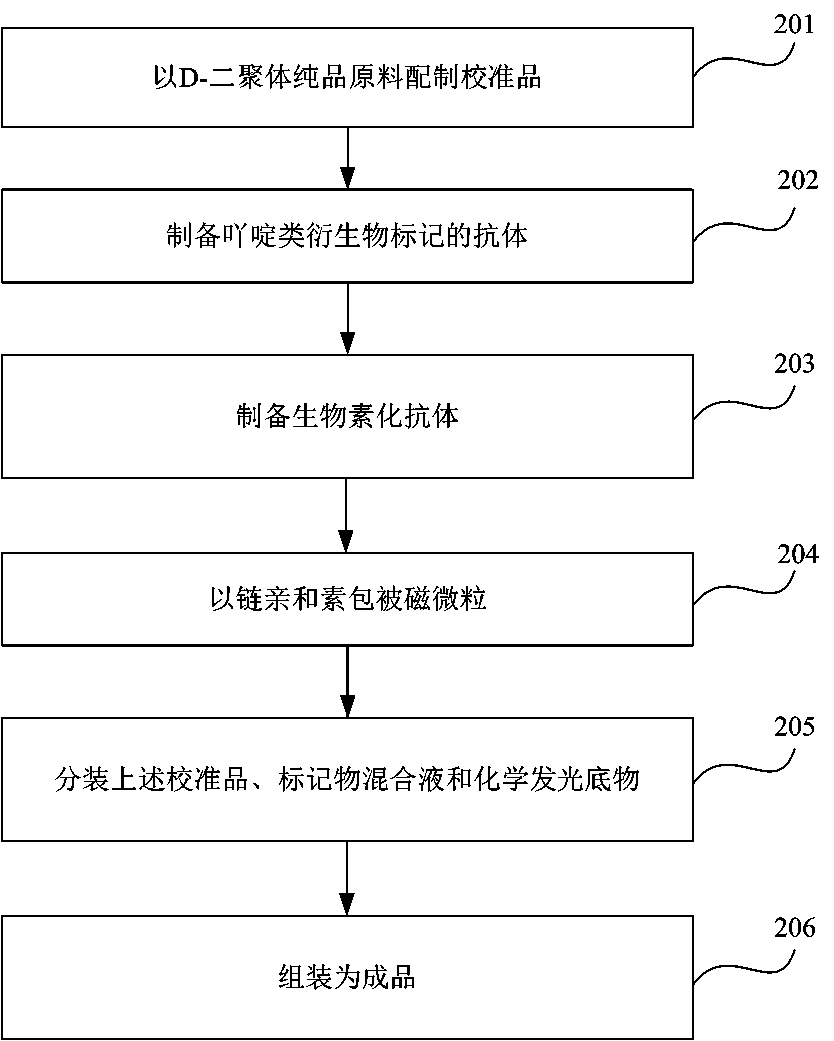
[0056] Example 2 Preparation of the D-dimer quantitative assay kit of the present invention
[0057] 1. Preparation of acridine lipid antibody
[0058] 1. Acridine-labeled monoclonal antibody diluent Buffer A:
[0059] Buffer A 0.01-0.1M Tris-HCl pH 7.6, mass ratio 0.1%-1% deb bovine serum albumin (BSA), volume ratio 0.2% PC-300;
[0060] 2. Select the concentration of acridine-labeled monoclonal antibody:
[0061] The working concentration of acridinium-labeled monoclonal antibody is greater than 0.5 μg / mL.
[0062] 2. Preparation of D-dimer calibrator
[0063] Dilute the D-dimer antigen with 0.01M PBS pH 7.2, BSA diluent with a mass ratio of 0.5%, and prepare five kinds of calibration products with standard concentrations of 0.2, 1, 5, 20, and 50 μg / mL.
[0064] 3. Preparation of antibodies labeled with acridine derivatives:
[0065] 1) Weigh an appropriate amount of D-dimer monoclonal antibody, and use 0.05mol / L pH 9.5 CB to adjust to 1mg / mL;
[0066] 2) Acridine lipi...
Embodiment 3
[0091] Example 3 Buffer Buffer
[0092] As an important component of the present invention, the buffer is a buffer for diluting biotinylated antibodies, antibodies labeled with acridine derivatives, and streptavidin-coated magnetic particles, so as to further reduce specific binding and improve the analytical sensitivity of reagents.
[0093] Buffer A: 0.01-0.1M Tris-HCl pH 7.6, mass ratio 0.1%-1%BSA, volume ratio 0.2% PC-300;
[0094] Buffer B: 0.01-0.1M 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) at pH 7.8, 5% mouse serum by volume, 0.5-2mg / mL sheep immunoglobulin G, 0.1%-5 by mass % trehalose, 0.5%-1% casein sodium salt by mass ratio, and 0.2% PC-300 by volume ratio.
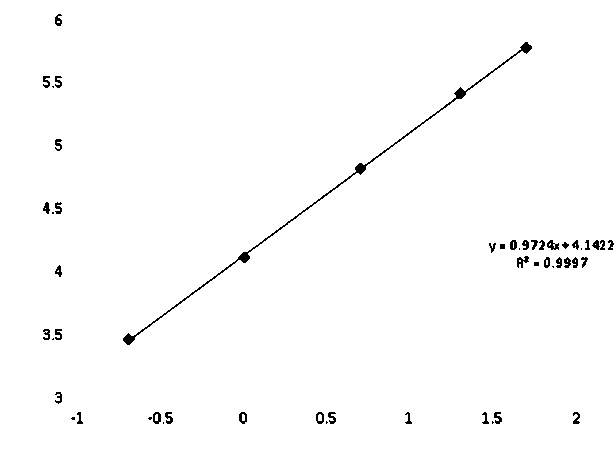
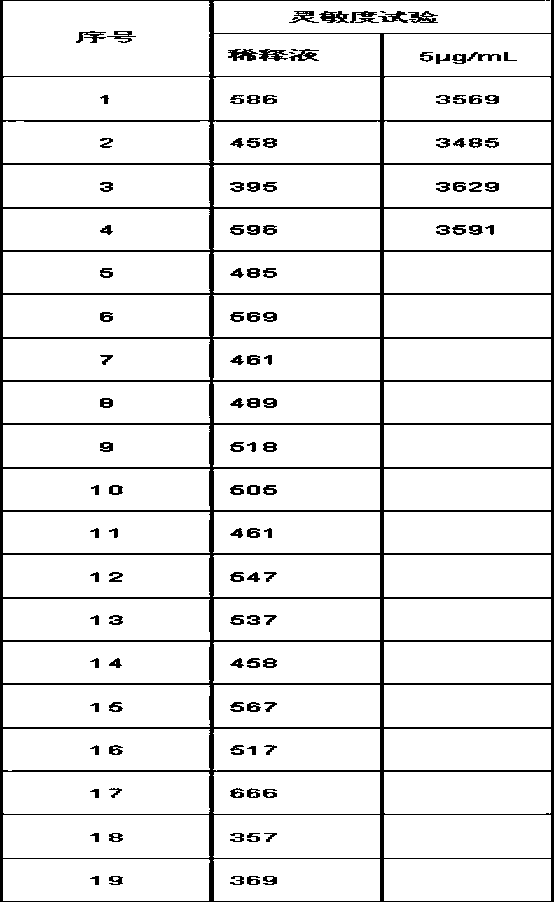
[0095] In one of the embodiments, trehalose can also be sucrose or fructose, and some experimental data are as follows:
[0096]
[0097] Analytical Sensitivity=2*(0 value RLU SD)*5 / (Calibrator RLU mean - 0 value RLU mean)
[0098] Use Buffer B as the diluent of acridinium ester-D-dimer antibody...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com