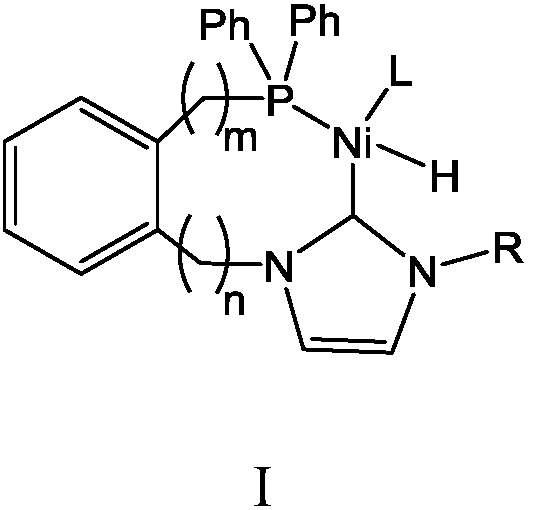
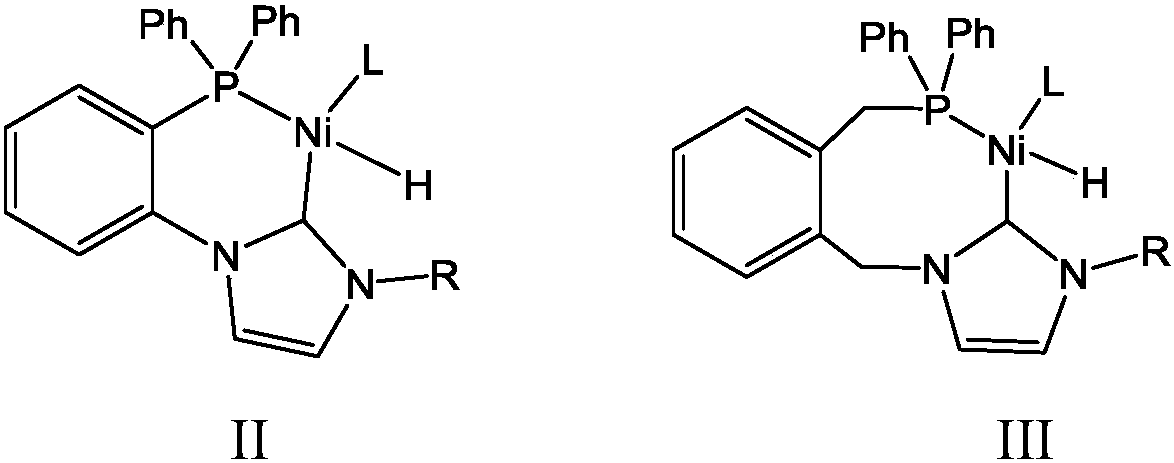
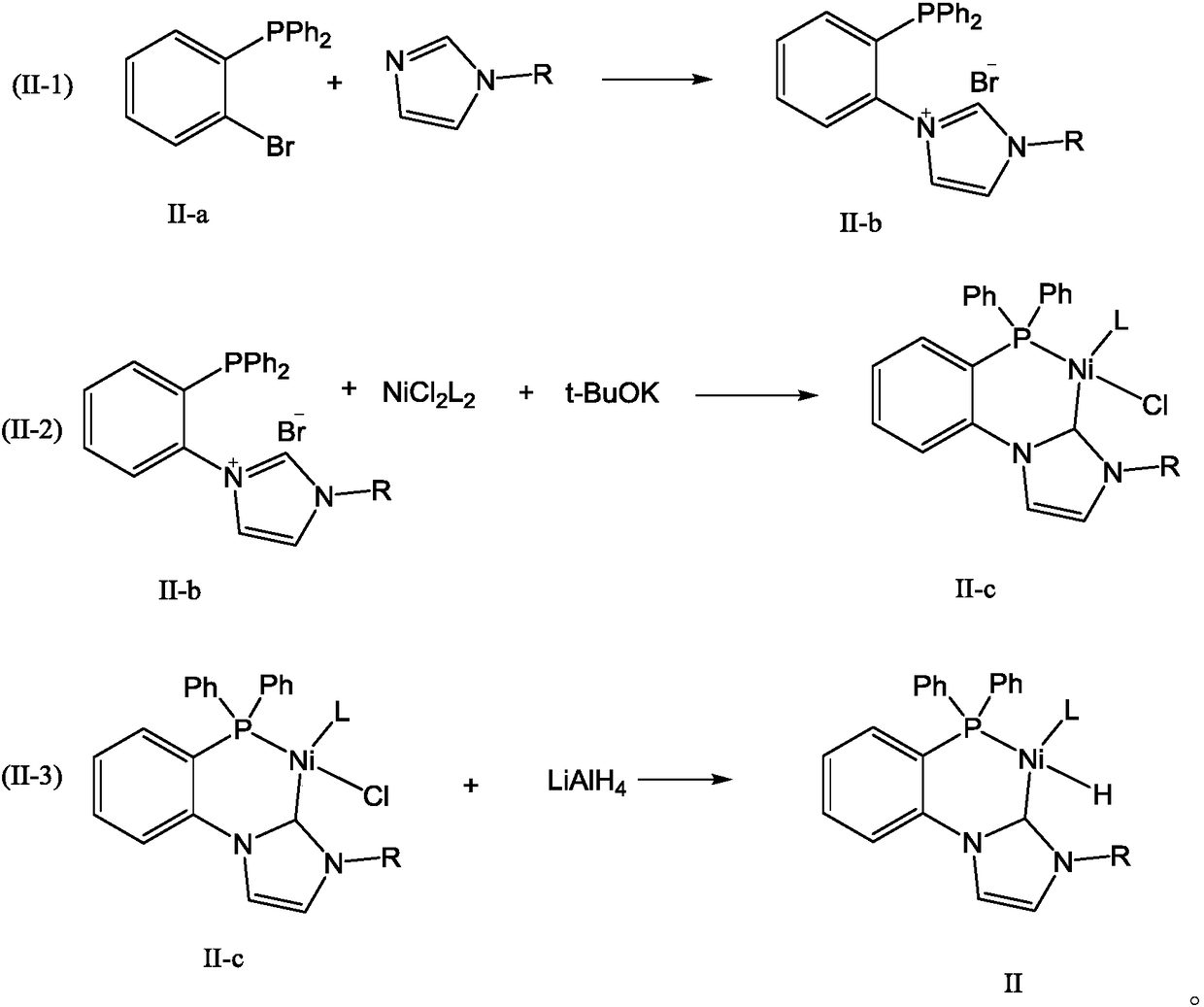
Olefin oligomerization catalyst as well as preparation method and use method thereof for olefin oligomerization
A catalyst and olefin technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the three wastes generation, easy deactivation, and catalysts are sensitive to water and oxygen impurities And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1) Preparation of catalyst
[0064]
[0065] (2-Bromophenyl)diphenylphosphine and 1.1 molar equivalent of methylimidazole were heated and stirred at 60°C for 1 h, cooled to room temperature, washed three times with dichloromethane, and filtered to obtain compound 1a with a yield of 85%. 1 H NMR (300MHz, CDCl3): δ = 3.72 (s, 3H), 7.71-7.89 (m, 16H), 8.92 (s, 1H). Mass spectrometry data: MS (EI) m / z (relative intensity): 423, 344, 160, 79.
[0066]
[0067] Under nitrogen protection, compound 1a and 1.1 molar equivalents of NiCl 2 (PPh 3 ) 2 , 1.5 molar equivalents of potassium tert-butoxide, mixed with tetrahydrofuran of the same mass as the reactants used, and reacted at -20°C for 2 hours. After the reaction, remove the tetrahydrofuran, add hexane, filter to obtain a hexane solution, and cool down to 0°C. After standing overnight, a solid crystallized out and was filtered to obtain compound 1b with a yield of 85%. 1H NMR (300MHz, CDCl3): δ=3.81(s, 3H), 5.06(...
Embodiment 2
[0077] (1) Preparation of catalyst
[0078]
[0079](2-Bromophenyl)diphenylphosphine and 1.2 molar equivalents of ethylimidazole were heated and stirred at 250°C for 4 h, cooled to room temperature, washed three times with dichloromethane, and filtered to obtain compound 2a with a yield of 90%. 1H NMR (300MHz, CDCl3): δ=1.29(t, 3H, J=8.0Hz), 4.12(q, 2H, J=8.0Hz), 7.71-7.89(m, 16H), 8.92(s, 1H) mass spectrum Analytical data: MS (EI) m / z (relative intensity) 439, 359, 174, 79.
[0080]
[0081] Under nitrogen protection, compound 2a and 1.2 molar equivalents of NiCl 2 (PPh 3 ) 2 , 1.2 molar equivalents of potassium tert-butoxide, mixed with tetrahydrofuran of the same mass as the reactants used, and reacted at 20°C for 4h. After the reaction, remove the tetrahydrofuran, add hexane, cool down to 10°C, leave it overnight, precipitate solids, and filter Compound 2b was obtained with a yield of 87%. 1H NMR (300MHz, CDCl3): δ=1.29(t, 3H, J=8.0Hz), 4.12(q, 2H, J=8.0Hz), 5.0...
Embodiment 3
[0091] (1) Preparation of catalyst
[0092]
[0093] (2-Bromophenyl)diphenylphosphine and 1.2 molar equivalents of n-octyl imidazole were heated and stirred at 150°C for 2 h, cooled to room temperature, washed three times with dichloromethane, and filtered to obtain compound 3a with a yield of 98%. 1H NMR (300MHz, CDCl3): δ=0.88(t, 3H, J=8.0Hz), 1.29-1.74(m, 12H,), 4.04(d, 2H, J=7.1Hz), 7.3-8.1(m, 16H), 8.95(s, 1H). Mass spectrometry data: MS (EI) m / z (relative intensity) 442,338,226,79.
[0094]
[0095] Under nitrogen protection, compound 3a and 1.2 molar equivalents of NiCl 2 (PPh 3 ) 2 , 1.2 molar equivalents of potassium tert-butoxide, mixed with tetrahydrofuran of the same mass as the reactants used, and reacted at -10°C for 2 hours. After the reaction, remove the tetrahydrofuran, add hexane, cool down to 2°C, and stand overnight to precipitate a solid. Compound 3b was obtained by filtration with a yield of 90%. 1H NMR (300MHz, CDCl3): δ=0.88(t, 3H, J=8.0Hz),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com