Preparation method of alpha-formyl-beta-formamido-propionitrile alkali metal salt
A technology of formylaminopropionitrile base and aminopropionitrile, which is applied in the field of preparation of α-formyl-β-formylaminopropionitrile alkali metal salt, can solve the problems of difficult control conditions, low conversion rate and potential safety hazards And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
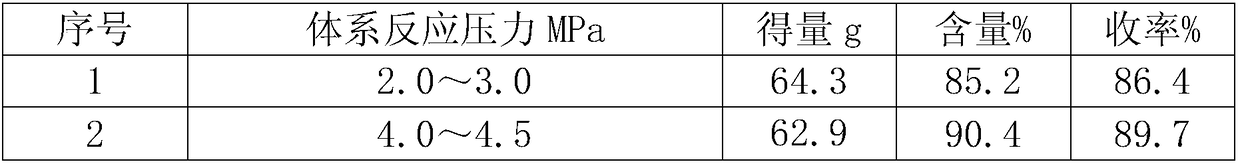
[0014] Add 284g of ethyl formate into a 500mL three-necked bottle, and slowly add 35g of solid sodium ethoxide at a rate of 1.2g / min at a certain temperature (see Table 1 for the specific selection of the temperature). Add 30g of β-aminopropionitrile dropwise at a speed of min, pour the above reaction solution into a 500mL autoclave, start stirring, heat to make the temperature rise to 65-70°C, the pressure range is 5.0-6.0MPa, and it will naturally drop to 20 after 10 hours of heat preservation. ℃ discharge, suction filtration, the filter cake was washed twice with 25g ethyl formate, and the filter cake was dried at 50 ℃ to obtain 65.8g of α-formyl-β-formylaminopropionitrile yellowish solid sodium salt with a content of 90.4 %, yield 93.8%.
[0015] Table 1: The selection of solid sodium ethylate addition temperature and the amount and yield of α-formyl-β-formylaminopropionitrile sodium salt
[0016] serial number
Embodiment 2
[0018] Add 284g of ethyl formate into a 500mL three-necked flask, slowly add 43.5g of solid sodium ethylate at a rate of 1.2g / min at -10 to 0°C, raise the temperature to 10°C, and then dropwise add 30g of β-amino at a rate of 1.2g / min For propionitrile, pour the above reaction solution into a 500mL autoclave, start stirring, and heat to make the temperature rise to a certain temperature (see Table 2 for the selection of temperature). ℃ discharge, suction filtration, the filter cake was washed twice with 25g ethyl formate, and the filter cake was dried at 60 ℃ to obtain the sodium salt of α-formyl-β-formylaminopropionitrile. The yield is shown in Table 2. The mother liquor obtained after filtration was rotary-evaporated to obtain ethanol and ethyl formate, and applied mechanically to ethyl formate to react, and the residue α-formyl-β-formylaminopropionitrile sodium salt was just dissolved in a small amount of water, and then recrystallized twice with 10g of acetone. 3 g of α-fo...
Embodiment 3
[0022] Add 200g of ethyl formate into a 500mL three-necked flask, slowly add 43.5g of solid sodium ethylate at a rate of 1.5g / min at -10 to 0°C, raise the temperature to 10°C, and then dropwise add 30g of β-amino at a rate of 1.5g / min For propionitrile, pour the above reaction solution into a 500mL autoclave, start stirring, heat to make the temperature rise to 65-70°C, and the pressure range is 5.0-6.0MPa, keep warm for 10 hours and naturally drop to 30°C to discharge, rotary steaming, The mixed solvent of ethanol and ethyl formate was distilled off and applied mechanically to the reaction of preparing ethyl formate to obtain 65.2 g of α-formyl-β-formylaminopropionitrile sodium salt with a yield of 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com