Iridium complex taking sulfo aromatic ring/aromatic heterocyclic phosphate compound as auxiliary ligand
A technology of iridium complexes and aromatic heterocycles, which is applied in the field of iridium complex light-emitting materials, can solve the problems of reducing device efficiency and carrier imbalance, and achieve the effects of improving electron mobility, simple preparation method, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Preparation of auxiliary ligand thiodipyridyl sodium phosphate aqueous solution
[0036] 2-bromopyridine and phosphorus trichloride (3:1) were refluxed in toluene under anaerobic conditions for two hours. Reflux for two hours under conditions, and stir in aqueous sodium hydroxide solution for two hours to obtain sodium thiodipyridylphosphate aqueous solution The yield reached 100%.
[0037] The above method can be used to prepare other thioaromatic / aromatic heterocyclic phosphoric acid compound sodium salt solutions:
[0038]
[0039]
[0040]
Embodiment 2
[0041] The preparation of embodiment 2 iridium complexes of the present invention
[0042] The main ligand 2-(2,4-difluorophenyl)pyridine and IrCl 3 Reflux 10 hours in ethoxyethanol solution with the ratio of 2:1, the chlorine bridge complex that obtains iridium by cold filtration; The crude product of the iridium complex was obtained by reflux in oxyethanol for two hours, and 14.70 g of pure product Sdpp 1 was obtained by column chromatography (yield: 91%). and further placed 5 g of Sdpp 1 in a quartz tube at 10 -5 Heating and sublimation purification under Pa vacuum conditions obtained 4.6 g of luminescent materials (sublimation rate 92%) that met the requirements for preparing devices. The response looks like this:
[0043]
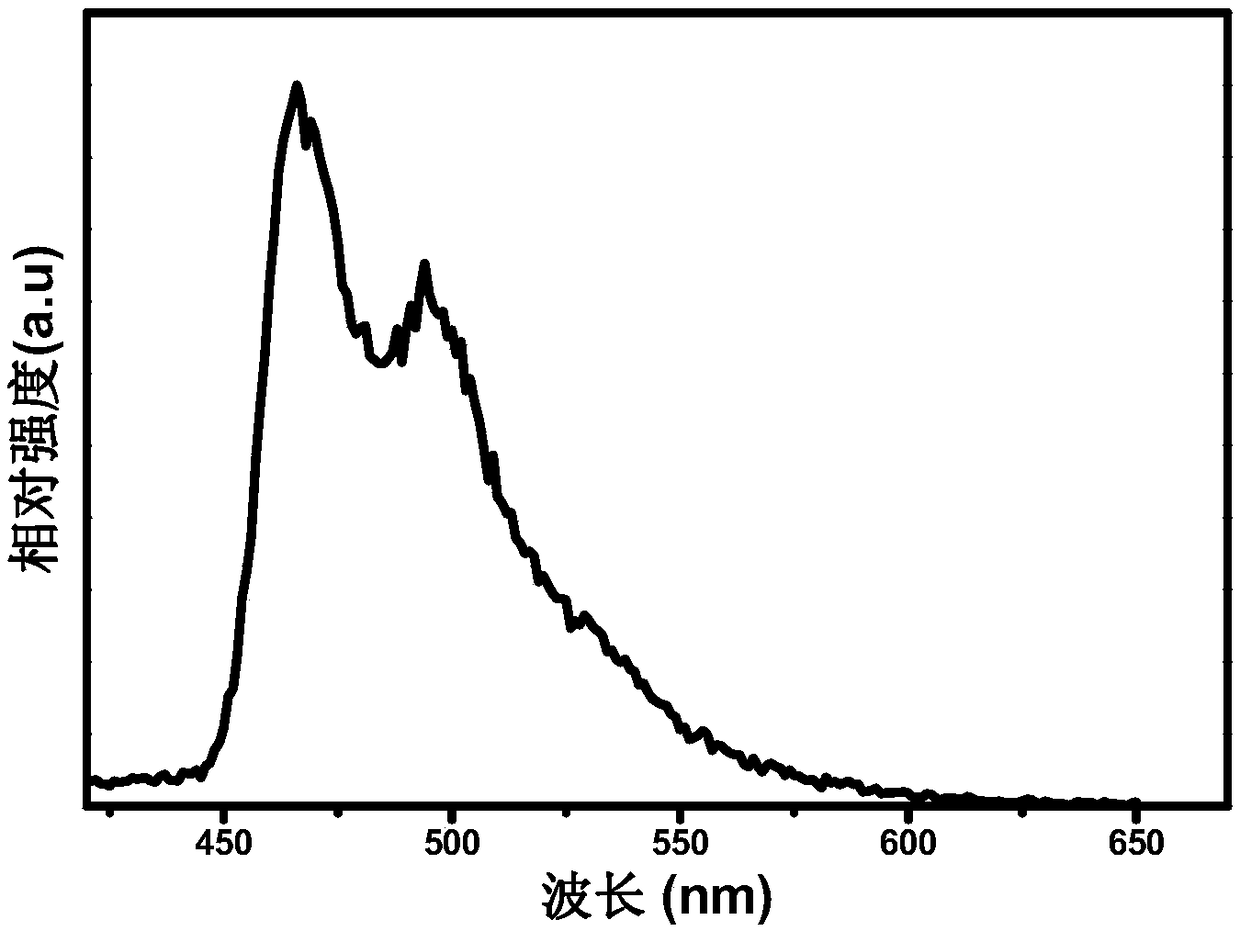
[0044] Gained iridium complex Sdpp 1 is analyzed as follows by proton nuclear magnetic resonance spectrum and high resolution mass spectrometry:
[0045] 1 H NMR (400MHz, CDCl 3 )δ8.70(d,1H),8.60(t,J=7.4Hz,3H),7.91(d,J=4.3Hz,2H),7.86(dd,J=4.3H...
Embodiment 3
[0054] Embodiment 3 Preparation of iridium complex Stpip 1 organic electroluminescent device
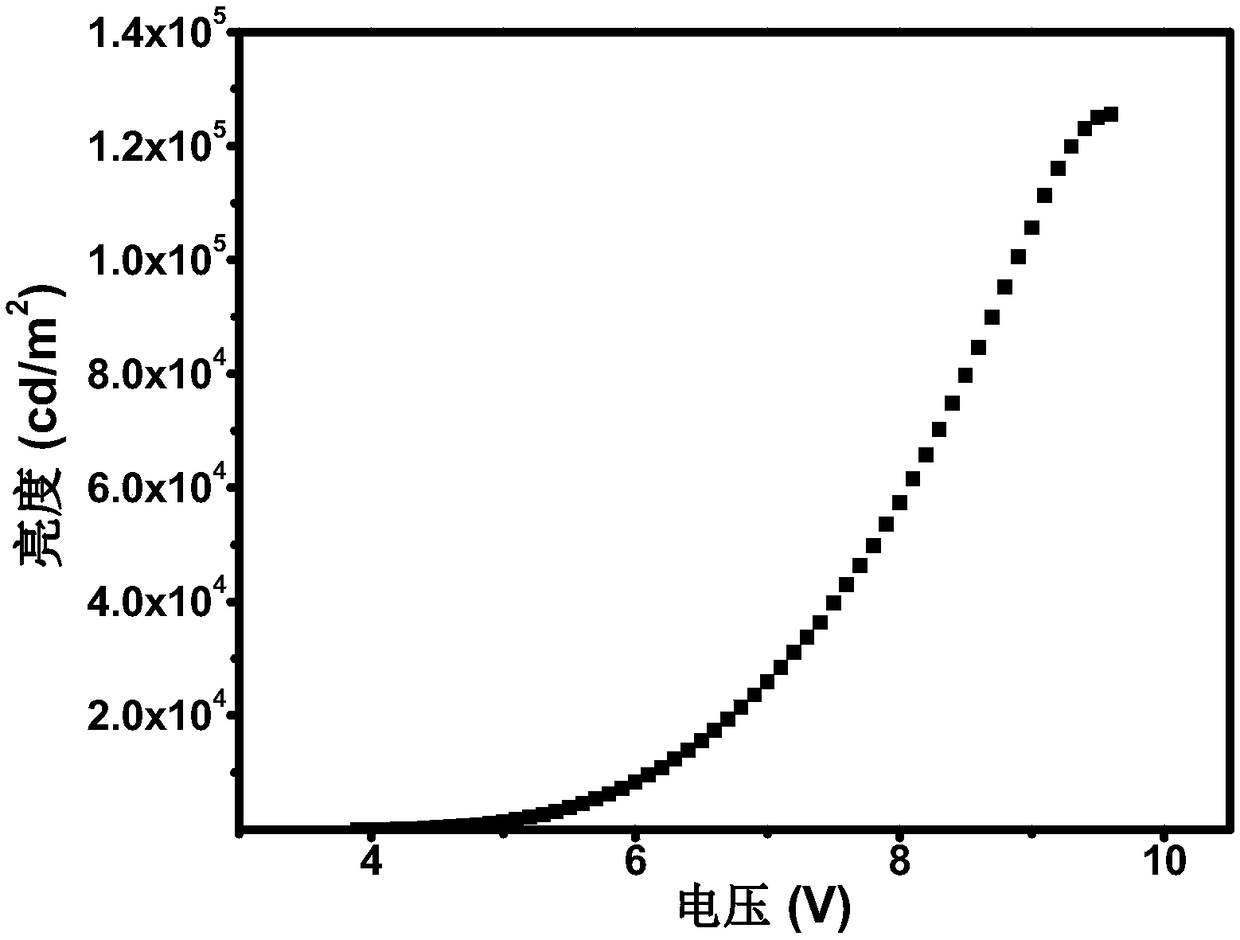
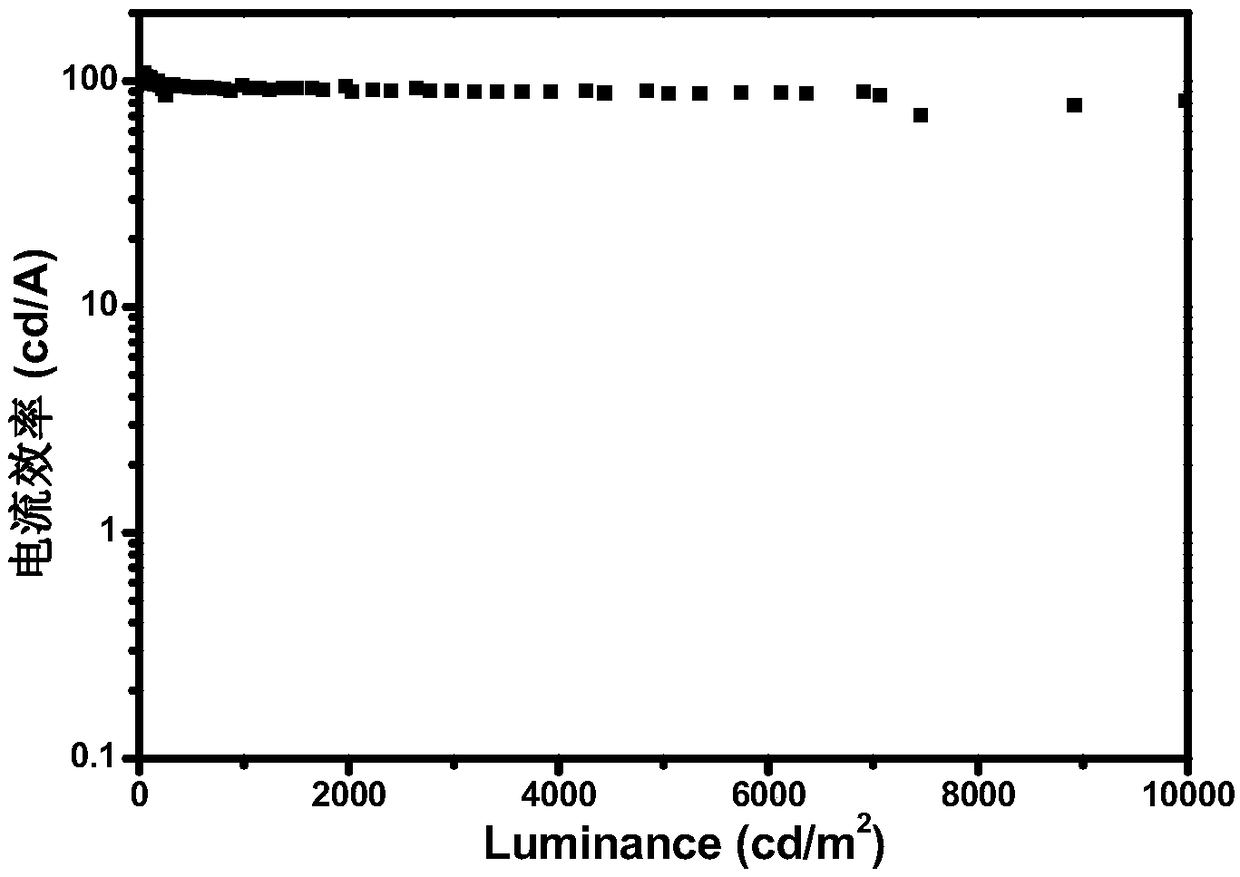
[0055] The preparation of the organic electroluminescent device of the present invention will be described below by taking Sdpp 1 as the luminescent center of the luminescent layer to prepare an organic electroluminescent device as an example. The structure of OLEDs device includes: substrate, anode, hole injection material, hole transport layer, organic light-emitting layer, electron transport layer, electron injection material and cathode. The substrate is glass, the anode is indium tin oxide, and the hole injection layer is 2,3,6,7,10,11-hexacyano-1,4,5,8,9,12-hexaazabenzene Phenanthrene HAT-CN (5nm), the evaporation rate is 0.05nm / s; the hole layer is made of 4,4'-cyclohexylbis[N,N-bis(4-methylphenyl)aniline TAPC material (50nm) , the evaporation rate is 0.05nm / s; the electron transport layer adopts 1,3,5-tris[(3-pyridyl)-3-phenyl]benzene TmPyPb (50nm), the evaporation rate is 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum brightness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
| Brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com