Fermenting preparation method of oosporein
A technology of oosporin and bacterial strain, which is applied in the field of oosporin fermentation and preparation, can solve the problems of unreachable industrialized production, high production cost, and low oosporin output, and improve cell concentration and product synthesis efficiency , the effect of increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
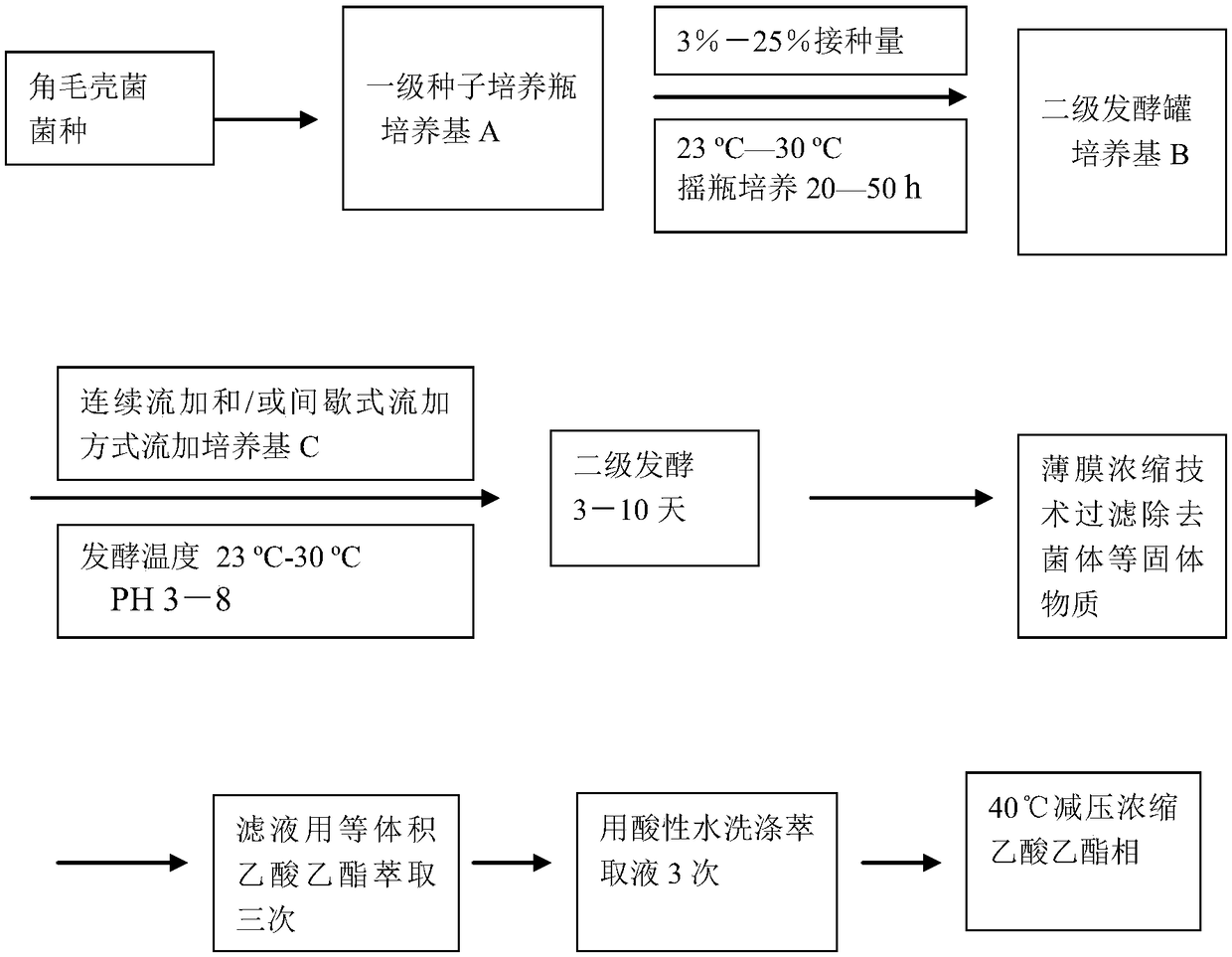
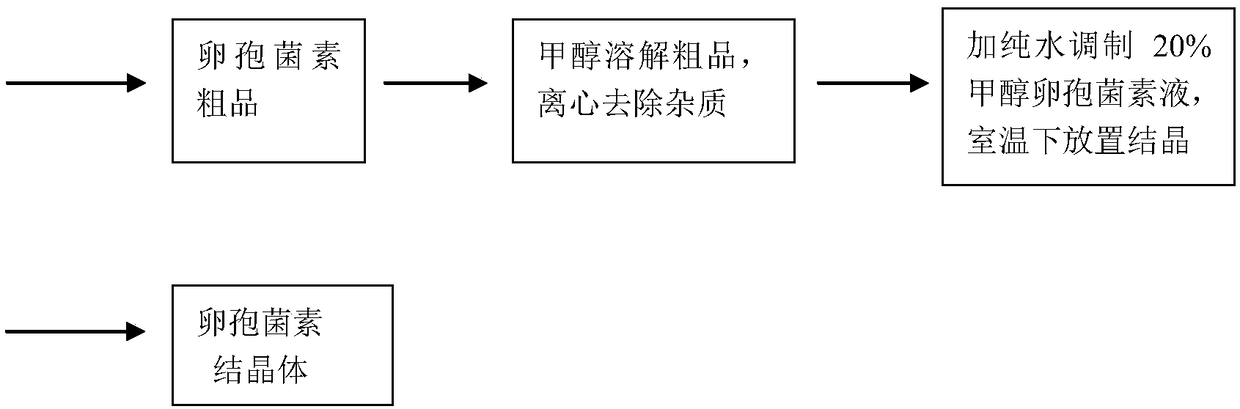
[0049] Use 10 1000mL Erlenmeyer flasks, each containing 300mL medium A (glucose 3.5%, peptone 1.0%, soluble starch 3.0%, peanut meal 1.0%, corn meal 2.2%, soybean meal 0.2%, potato extract juice 1.5%, Magnesium sulfate 0.05%, potassium dihydrogen phosphate 0.05%), sterilized at 120°C for 30 minutes, inoculated with activated Chaetomium cupreum (Chaetomium cupreum) CH-1 spore liquid after cooling, and cultured in shake flasks at 25°C for 42 hours. Inoculate the cultured strain solution into a 100L fermenter with 50L sterile medium B in a 10% inoculum, (medium B: 3.0% starch, 1.0% glycerol, 1.0% lactose, 2.5% sucrose, sulfuric acid Ammonium 0.05%, glucose 3.0%, peptone 1.5%, soybean powder hydrolyzate 1.0%, peanut cake powder 2.5%, soybean protein 2.0%, fermented soybean meal 1.0%, corn flour 1.5%, yeast powder 0.1%, dipotassium hydrogen phosphate 0.2 %, iron sulfate 0.01%, magnesium sulfate 0.01%, sodium chloride 0.1%), for fermentation production. After the strains were inocu...
Embodiment 2
[0053] Use 10 1000mL triangular flasks, each containing 300mL medium A (2.5% glucose, 1.5% peptone, 3.0% soluble starch, 1.5% peanut meal, 2.2% corn meal, 0.2% soybean meal, 1.5% potato extract juice, Magnesium sulfate 0.05%, potassium dihydrogen phosphate 0.05%), sterilized at 120°C for 30 minutes, inoculated with activated Chaetomium cupreum (Chaetomium cupreum) CH-1 spore liquid after cooling, and cultured in shake flasks at 25°C for 42 hours. Inoculate the cultured strain solution into a 100L fermenter with 50L sterilized medium B in a 10% inoculum, (medium B: 3.0% starch, 1.0% glycerol, 2.0% lactose, 2.5% sucrose, sulfuric acid Ammonium 0.05%, glucose 4.0%, peptone 1.5%, soybean powder hydrolyzate 2.0%, peanut cake powder 2.5%, soybean protein 2.0%, fermented soybean meal 1.0%, corn flour 2.5%, yeast powder 0.1%, dipotassium hydrogen phosphate 0.2 %, iron sulfate 0.01%, magnesium sulfate 0.01%, sodium chloride 0.1%), for fermentation production. After the strains were in...
Embodiment 3
[0057]Use 10 1000mL Erlenmeyer flasks, each containing 300mL medium A (glucose 3.5%, peptone 1.0%, soluble starch 3.0%, peanut meal 1.0%, corn meal 2.2%, soybean meal 0.2%, potato extract juice 1.5%, Magnesium sulfate 0.05%, potassium dihydrogen phosphate 0.05%), sterilized at 120°C for 30 minutes, inoculated with activated Chaetomium cupreum (Chaetomium cupreum) CH21-2-20 spore liquid after cooling, and cultured in shake flask at 25°C for 42 Hour. Inoculate the cultured strain solution into a 100L fermenter with 50L sterile medium B inside at an inoculum size of 10%, (medium B: starch 3.0%, glycerol 1.5%, lactose 2.5%, sucrose 2.5%, sulfuric acid Ammonium 0.05%, glucose 3.5%, peptone 1.5%, soybean powder hydrolyzate 2.5%, peanut cake powder 3.5%, soybean protein 3.5%, fermented soybean meal 1.0%, corn flour 1.5%, yeast powder 0.1%, dipotassium hydrogen phosphate 0.3 %, iron sulfate 0.01%, magnesium sulfate 0.01%, sodium chloride 0.2%), for fermentation production. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com