Preparation method for high-purity edoxaban intermediate
A technology for edoxaban and intermediates, which is applied in the field of preparation of edoxaban intermediates, can solve the problems of high price, unfavorable industrialized production, potential safety hazards, etc., and achieves the effects of low production cost and large-scale industrialized production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
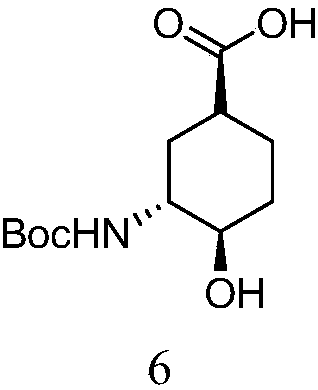
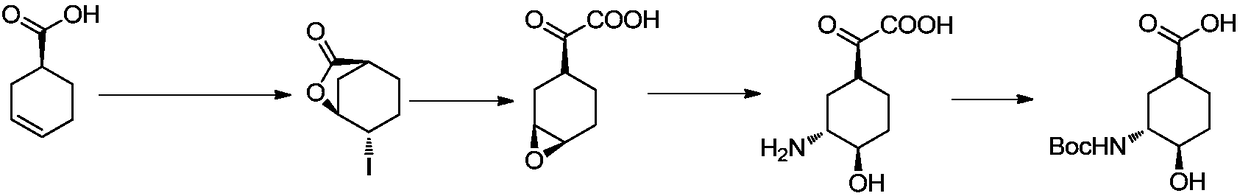
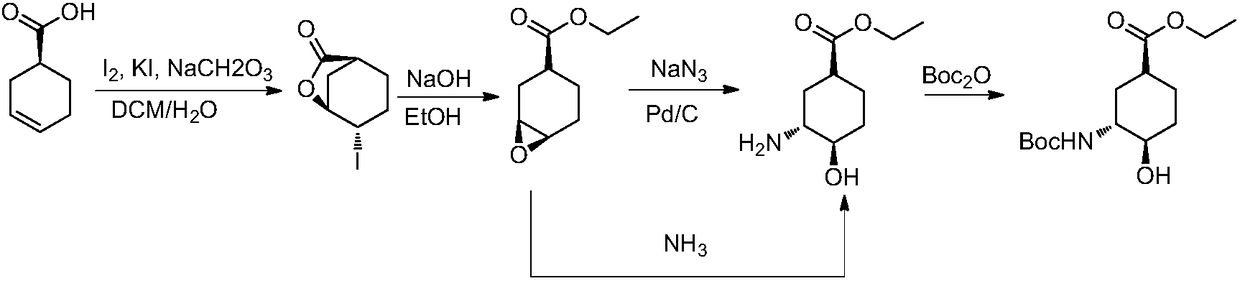
[0035] Embodiment 1, the preparation of (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxyl-cyclohexanecarboxylic acid
[0036]
[0037] (1) Preparation of 4-iodo-3-cyclohexylcarboxylate
[0038] Dissolve 200g of cyclohexene-1-carboxylic acid (1) in 600mL of dichloromethane, add 200g of sodium bicarbonate, 350g of potassium iodide, and 500mL of water under ice-cooling. After 10 minutes, warm up to room temperature and add 500g of iodine. After 2 hours, , adding sodium thiosulfate aqueous solution (2N, 300mL), after half an hour, separate the layers, extract the aqueous phase once with 500mL dichloromethane, combine the organic phases, dry, and spin dry to obtain 460g white solid (2).
[0039] (2) Preparation of 7-oxabicyclo[4.1.0]heptane-3-carboxylic acid methyl ester
[0040] Add 30 g of 4-iodo-3-cyclohexanecarboxylic acid lactone into 120 mL of methanol, stir at room temperature for 10 minutes, add sodium hydroxide (50 mL, 2.5 N), stop the reaction after 3 hours, add 300 ...
Embodiment 2
[0047] Embodiment 2, the preparation of (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxyl-cyclohexanecarboxylic acid
[0048] In 187.5 mL of phosphate buffer solution with pH=8.0, add 50 g of 3-tert-butoxycarbonylamino-4-hydroxy-cyclohexanecarboxylic acid methyl ester prepared in step (4) of Example 1, 1 g of papain, 562.5 mL Toluene, after stirring at 50°C for 48 hours, terminate the reaction, separate the liquids, wash the water phase once with ethyl acetate, continue to add an equal volume of ethyl acetate to the water phase, cool to 0-10°C, and add a certain amount of hydrochloric acid ( 6N), adjust the pH to 2. The liquid was separated, and methyl tert-butyl ether (250 mL) was added to the aqueous phase to extract twice, and the organic phases were combined, dried, and spin-dried to obtain 21 g of a colorless solid, de=99%.
Embodiment 3
[0049]Example 3, (1S, 3R, 4R) Screening of enzymes in the process of (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxyl-cyclohexanecarboxylic acid
[0050] 10g of ethyl 3-tert-butoxycarbonylamino-4-hydroxy-cyclohexanecarboxylate, 40mL of PBS (PH=7.2), 10mL of methyl tert-butyl ether were added to a 100mL three-necked flask, and 0.2g of different hydrolysates were added, The reaction was carried out at 30° C. for 48 hours, and the reaction results are shown in Table 1.
[0051] The hydrolysis of different hydrolase catalyzed formula 5 compounds of table 1
[0052]
[0053] The results showed that the effects of alkaline protease, pepsin, trypsin and papain were better, and the de values were all greater than 98%. In terms of overall yield, when the hydrolase is selected from alkaline protease, the effect is the best.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com