Naphthoquinone compounds, preparation thereof and application to hypoglycemic product preparation
A compound and hypoglycemic technology are applied in the field of natural medicinal chemistry to achieve the effects of good hypoglycemic activity, novel structure and good medicinal and dietary effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
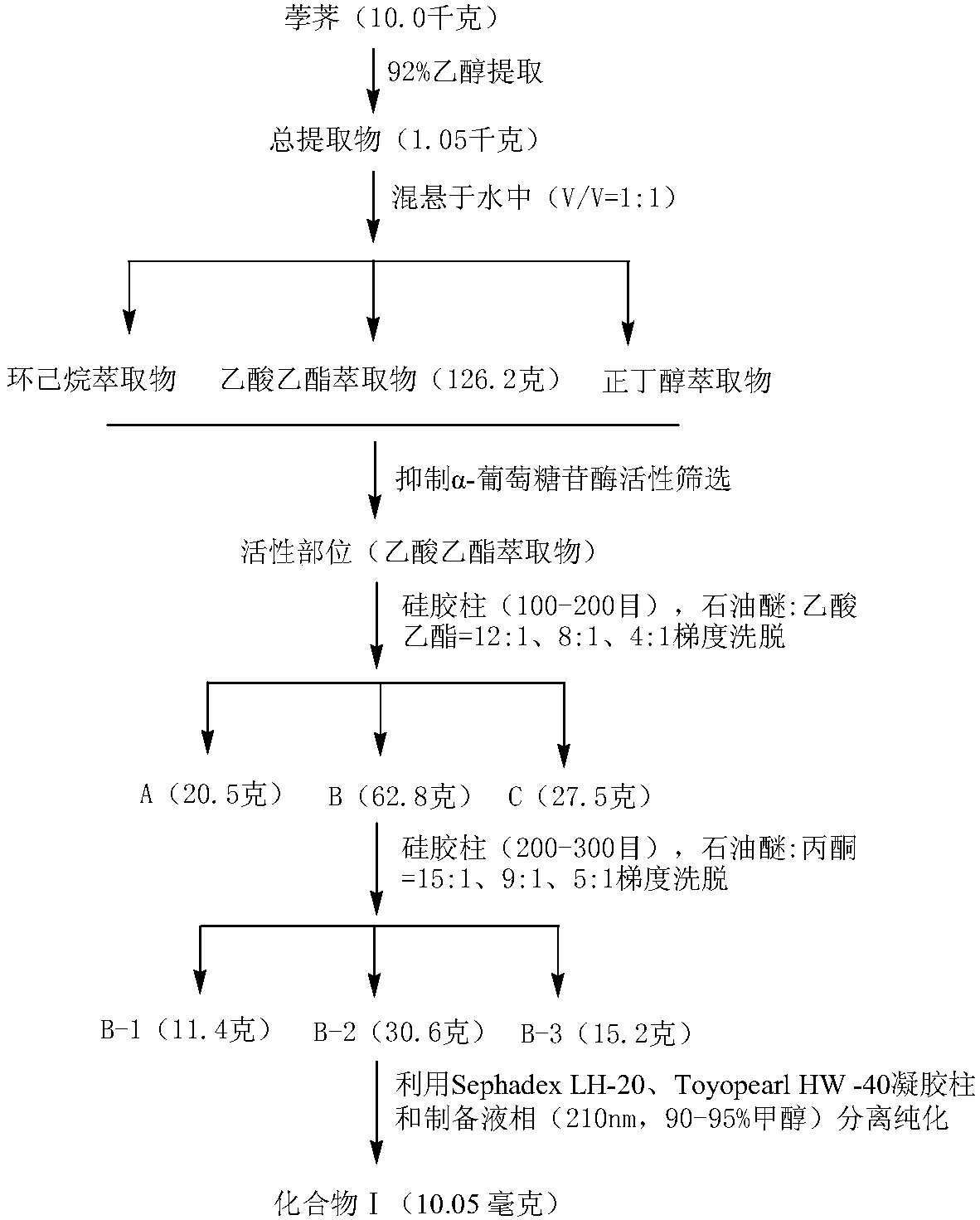
[0044] (1) Grind dried water chestnuts (10.0 kilograms) into small pieces (0.8 centimeters), put them in a 92% ethanol solution and soak them for 16 hours, then carry out heat reflux extraction for 3 times, each time for 3 hours, the extracts are combined and Filtrate, and dry the filtrate at low temperature (53° C.) until it has no alcohol smell, and obtain the total extract of water chestnuts (1.05 kg);
[0045] (2) Suspend the total water chestnut extract in water (V / V=1:1), knead and dissolve (i.e. fully dissolve), let stand for 24 hours, filter to obtain the supernatant; Ester and n-butanol are extracted, and the solvent is evaporated to dryness to obtain cyclohexane extract (46.5 grams), ethyl acetate extract (126.2 grams) and n-butanol extract (258.6 grams);
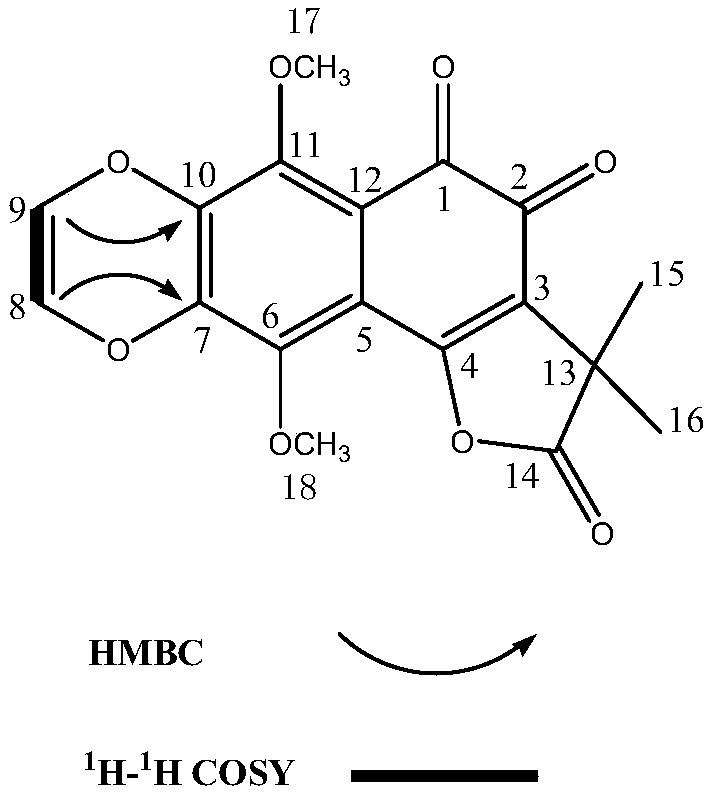
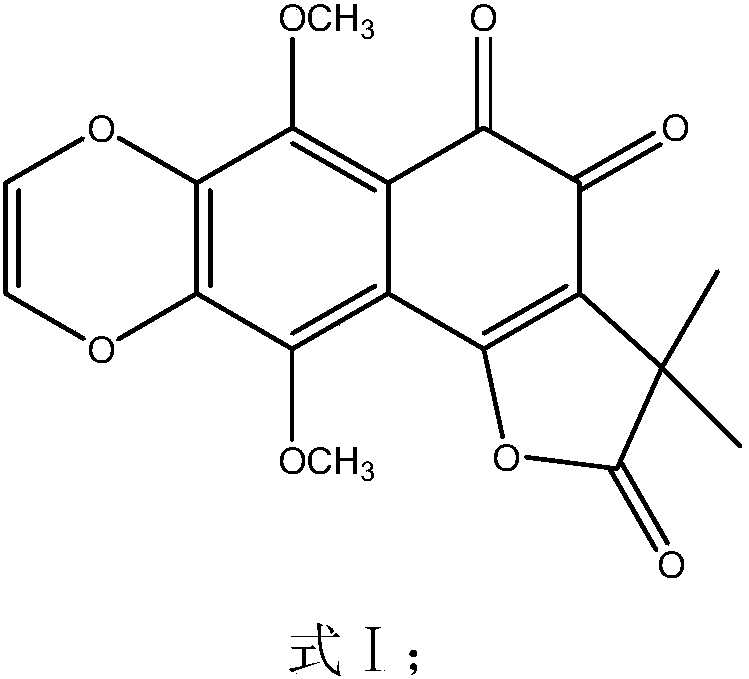
[0046] (3) The extract obtained in step (2) is subjected to an activity screening experiment for inhibiting α-glucosidase (see the activity screening part of Example 4 for details), and the results show that the e...
Embodiment 2
[0055] (1) Crush the dried water chestnuts into small pieces (0.5 cm), place them in a 95% ethanol solution and soak them for 18 hours, then carry out heat reflux extraction for 3 times, each time for 2 hours, the extracts are combined and filtered, and the filtrate is low-temperature (55° C.) drying to no alcohol smell to obtain the total extract of water chestnuts;
[0056] (2) Suspend the total water chestnut extract in water (V / V=1:1), knead and dissolve (i.e. fully dissolve), let stand for 22 hours, filter to obtain the supernatant; Ester, n-butanol are extracted, and the solvent is evaporated to dryness to obtain cyclohexane extract, ethyl acetate extract and n-butanol extract respectively;
[0057] (3) Dissolve the ethyl acetate extract prepared in step (2) with petroleum ether, and carry out silica gel column chromatography (wet column, 100-200 mesh); use petroleum ether-ethyl acetate elution system according to petroleum ether The volume ratio of ether-ethyl acetate ...
Embodiment 3
[0062] (1) Crush the dried water chestnuts into small pieces (1.0 cm), put them in a 90% ethanol solution and soak them for 12 hours, then carry out heat reflux extraction for 3 times, each time for 2.5 hours, the extracts are combined and filtered, and the filtrate Dry at low temperature (50°C) until there is no alcohol smell to obtain the total water chestnut extract;
[0063] (2) Suspend the total water chestnut extract in water (V / V=1:1), knead and dissolve (i.e. fully dissolve), let stand for 20h, filter to obtain the supernatant; Ester, n-butanol are extracted, and the solvent is evaporated to dryness to obtain cyclohexane extract, ethyl acetate extract and n-butanol extract respectively;
[0064] (3) Dissolve the ethyl acetate extract prepared in step (2) with petroleum ether, and carry out silica gel column chromatography (wet column, 100-200 mesh); use petroleum ether-ethyl acetate elution system according to petroleum ether The volume ratio of ether-ethyl acetate is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com