Micron-sized barium sulfate micro-sphere synthesis method
A synthesis method, barium sulfate technology, applied in chemical instruments and methods, calcium/strontium/barium compounds, calcium/strontium/barium sulfate, etc. The efficiency is not very high, and the effect of strong controllability of product shape, high yield and good particle size uniformity is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0034](1) Put the three-neck flask with a stirring device into a constant temperature oil bath, add 1.0mol / L BaCl 2 2H 2 O solution, followed by EDTA with Ba 2+ Add 1.0mol / L EDTA·2Na complexing agent at a molar ratio of 1.2:1, and control the temperature at 20°C to form a stable complex solution;
[0035] (2) Regulate step (1) complex solution pH to 7-10 with sodium hydroxide solution, after pH value is stable, according to S 2 o 8 2- with Ba 2+ Add 1.0mol / L Na at a molar ratio of 1:1 2 S 2 o 8 , after the reaction is complete, a white turbid solution with a pH of 5 is obtained;
[0036] (3) Stand still for 1 day, after centrifugation, wash with deionized water until the pH is neutral, and dry the obtained solid phase separation at 120° C. for 12 hours to obtain barium sulfate spherical particles with a yield of more than 90%.
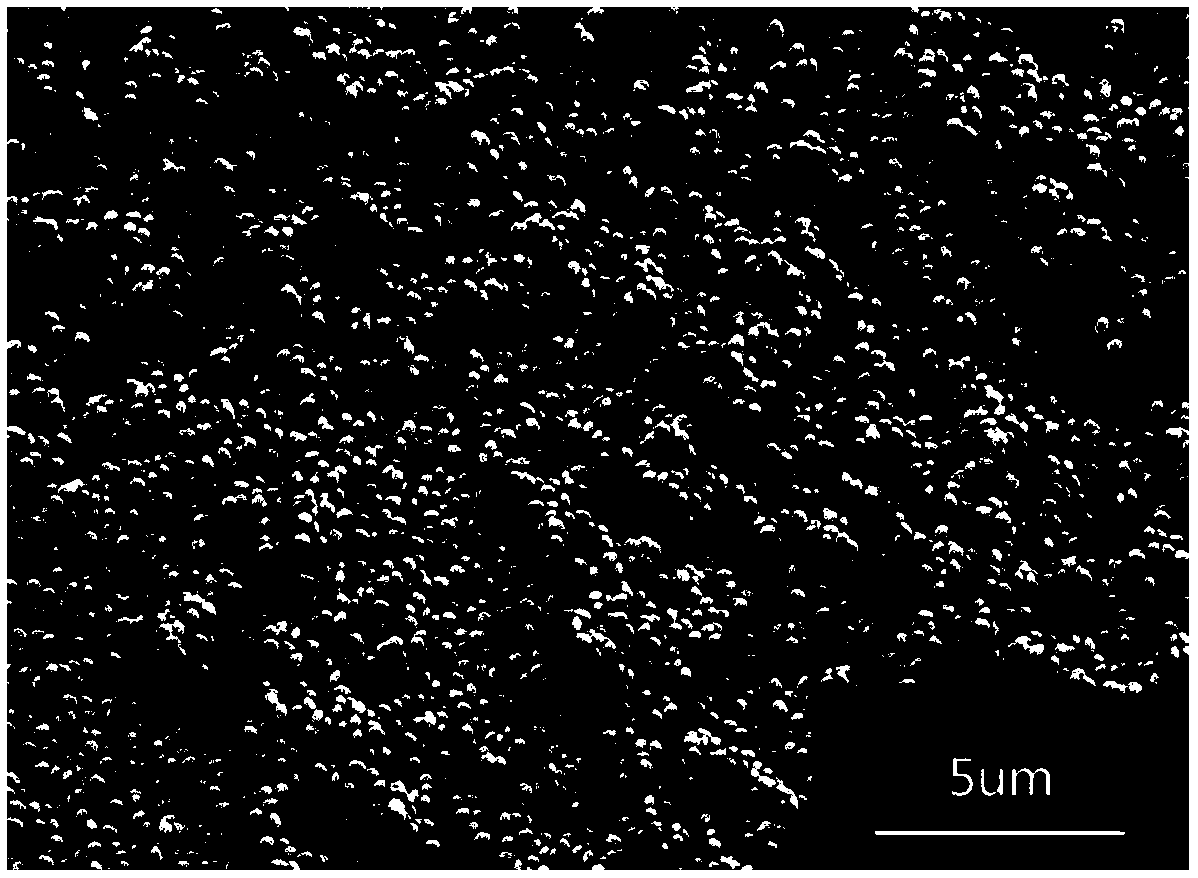
[0037] Observe above-mentioned obtained barium sulfate sample with ZEISS-SUPRA55 type field emission scanning electron microscope (Scanning e...
Embodiment 3
[0039] (1) Put the three-neck flask with a stirring device into a constant temperature oil bath, add 1.0mol / L BaCl 2 2H 2 O solution, followed by EDTA with Ba 2+ Add 1.0mol / L EDTA·2Na complexing agent at a molar ratio of 2:1, and control the temperature at 30°C to form a stable complex solution;
[0040] (2) Regulate the pH of the complex solution in step (1) to 7-10 with sodium hydroxide solution, after the pH value is stable, add excess Na 2 S 2 o 8 , after the reaction is complete, a white turbid solution with a pH of 2 is obtained;
[0041] (3) Stand still for 1 day, after centrifugation, wash with deionized water until the pH is neutral, and dry the obtained solid phase separation at 120° C. for 12 hours to obtain barium sulfate microspheres with a yield of 90%.
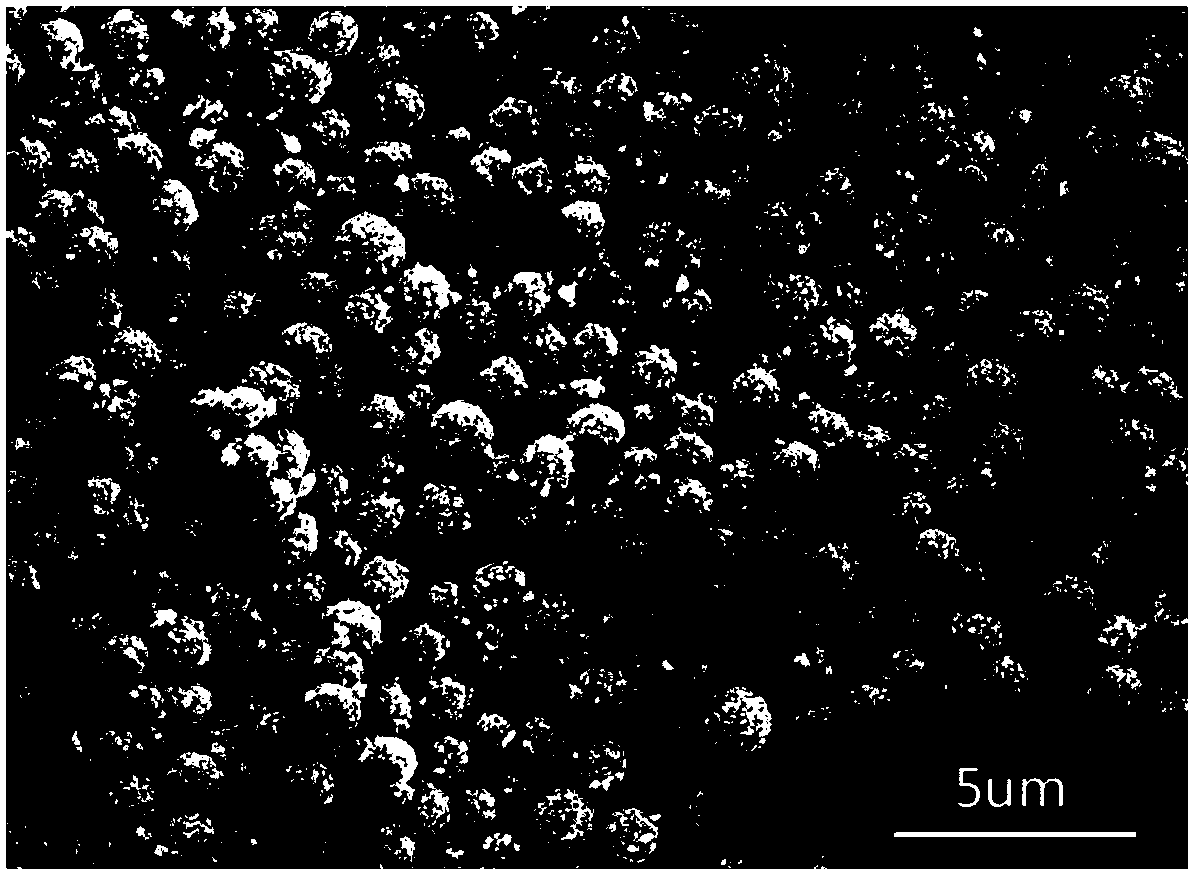
[0042] Observe above-mentioned obtained barium sulfate sample with ZEISS-SUPRA55 type field emission scanning electron microscope (Scanning electron microscope, SEM), as image 3 shown, from image 3 It c...
Embodiment 4
[0044] (1) Put the three-neck flask with a stirring device into a constant temperature oil bath, add 1.0mol / L BaCl 2 2H 2 O solution, followed by EDTA with Ba 2+ Add 1.0mol / L EDTA·2Na complexing agent at a molar ratio of 2:1, and control the temperature at 95°C to form a stable complex solution;
[0045] (2) Regulate the pH of the complex solution in step (1) to 8-9 with sodium hydroxide solution, after the pH value is stable, add excess K 2 S 2 o 8 , after the reaction is complete, a white turbid solution with a pH of 4 is obtained;
[0046] (3) Stand still for 1 day, after centrifugation, wash with deionized water until the pH is neutral, and dry the obtained solid phase separation at 120° C. for 12 hours to obtain barium sulfate microspheres with a yield of 93%.
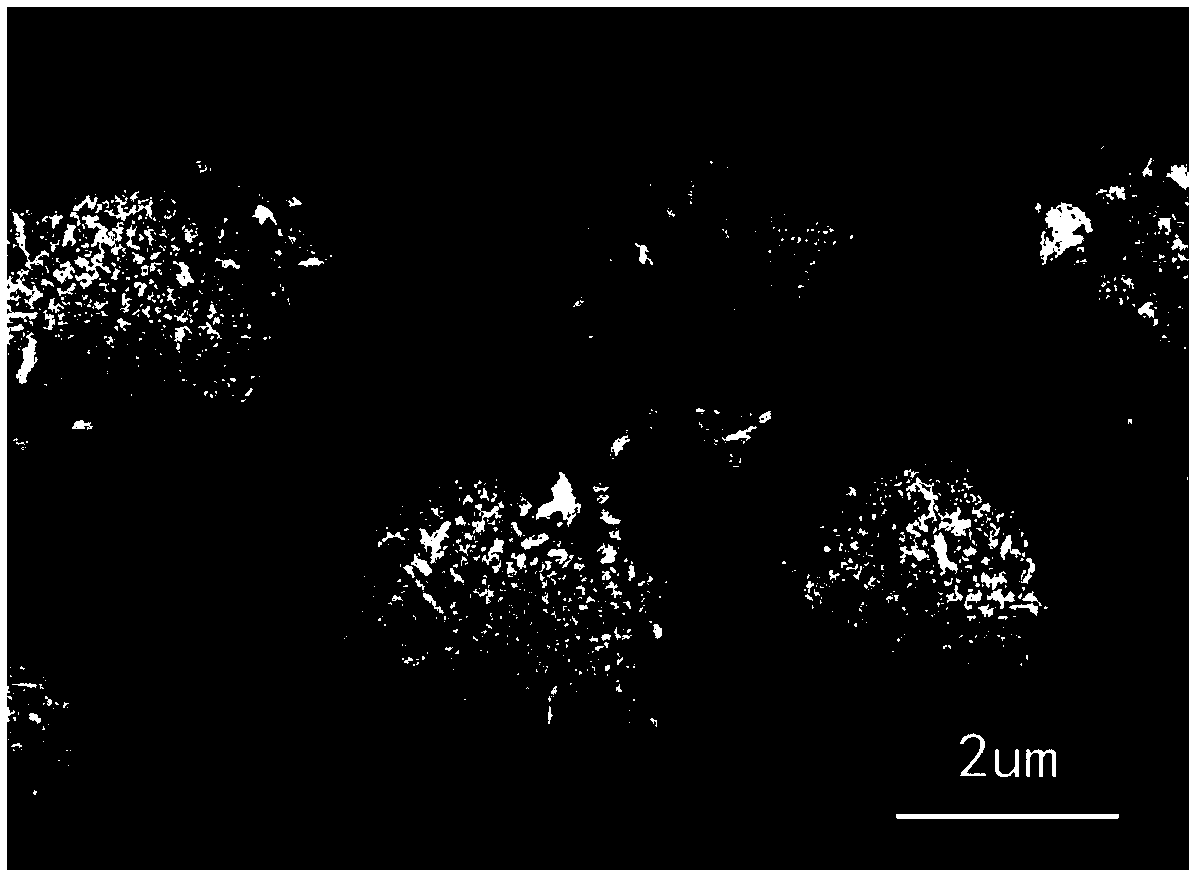
[0047] Observe above-mentioned obtained barium sulfate sample with ZEISS-SUPRA55 type field emission scanning electron microscope (Scanning electron microscope, SEM), as Figure 4 shown, from Figure 4 It c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com