O-substituted hydroxylamine hydrochloride and preparation method thereof
A hydroxylamine hydrochloride and substituent technology, applied in the field of O-substituted hydroxylamine hydrochloride and its preparation, can solve the problems of preparing O-sulfonylhydroxylamine hydrochloride, having too much waste acid, being unsuitable for industrial production, and the like, and achieving The effect of short reaction time, high yield and high versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
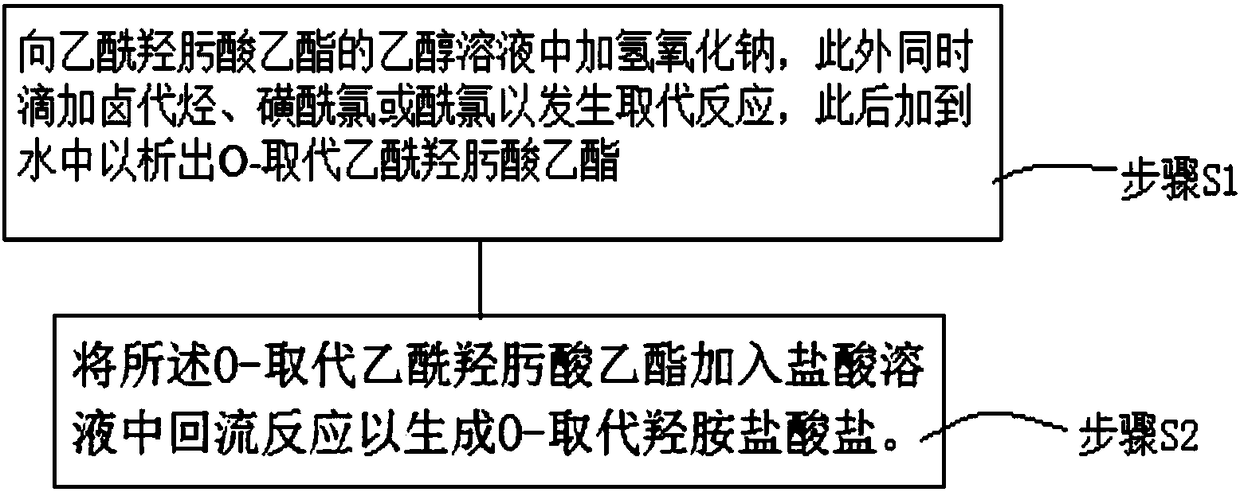
[0032] Such as figure 1 As shown, the preparation method of O-substituted hydroxylamine hydrochloride according to the embodiment of the present invention includes:
[0033] Step S1, adding sodium hydroxide to the ethanol solution of ethyl acetylhydroxamic acid, and simultaneously adding halogenated hydrocarbons, sulfonyl chlorides or acid chlorides dropwise until the reaction is complete, adding the reactant to water to precipitate O-substituted acetylhydroxamic acid ethyl ester.
[0034] Specifically, the molar ratio of ethyl acetylhydroxamate: sodium hydroxide: halogenated hydrocarbon or sulfuryl chloride or acid chloride is 1:(1.1-1.5):(1.2-1.5).
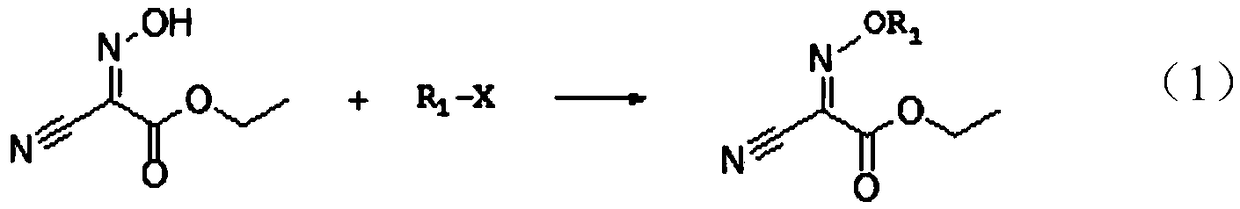
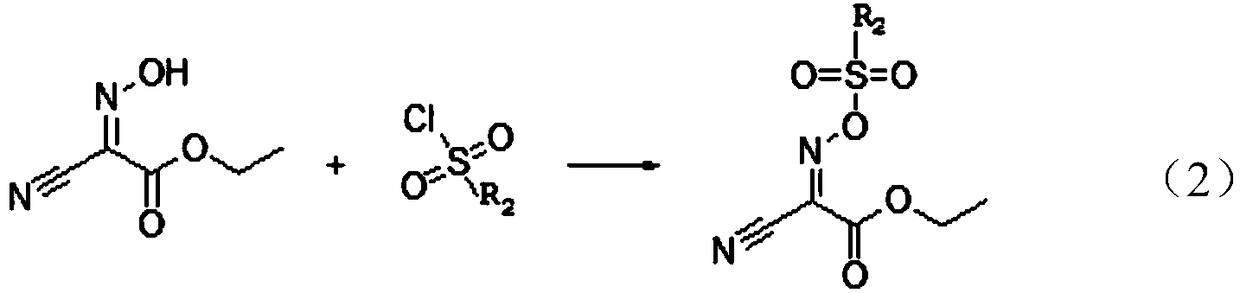
[0035] Further, the substituent on the oxygen is an alkyl group or an acyl group.
[0036] When the substituent on the oxygen is an alkyl group, add a halogenated hydrocarbon to the ethanol solution of ethyl acetylhydroxamate, wherein the halogenated hydrocarbon can be bromoethane, 2-bromopropane, 3-bromopropene, tert-butyl O...
Embodiment 1
[0066] (1) Preparation of compound O-allyl acetylhydroxamate ethyl ester
[0067] Take a 1L reaction bottle and add ethyl acetylhydroxamate (142g, 1.0mol, 1.0eq) and ethanol (426mL, 3P) to form an ethanol solution of ethyl acetylhydroxamate, and add it to the ethanol solution of ethyl acetylhydroxamate Add sodium hydroxide (44g, 1.1mol, 1.1eq), then dropwise add 3-bromopropene (145.2g, 1.2mol, 1.2eq), magnetically stir to speed up the reaction, control the reaction temperature between 15-25°C, add dropwise Complete and keep the temperature between 15-25°C for 2 hours, add the reaction solution to water (426mL, 3P), filter the precipitated solid with suction, wash the obtained filter cake with ethanol, dry the obtained solid to obtain 154.85gO - Ethyl allyl acetyl hydroxamate, the yield is 85%.
[0068] (2) Preparation of compound O-allyl hydroxylamine hydrochloride
[0069] Add ethyl O-allyl acetylhydroxamate (154.85g, 0.85mol, 1eq) into a solution of 3mol / L hydrochloric aci...
Embodiment 2
[0073] (1) Preparation of compound O-tert-butyl acetylhydroxamate ethyl ester
[0074] Take a 1L reaction bottle and add ethyl acetylhydroxamate (142g, 1.0mol, 1.0eq) and ethanol (426mL, 3P) to prepare an ethanol solution of ethyl acetylhydroxamate, and add it to the ethanol solution of ethyl acetylhydroxamate Add sodium hydroxide (44g, 1.1mol, 1.1eq), then dropwise add tert-butyl bromide (164.4g, 1.2mol, 1.2eq), magnetically stir to speed up the reaction, control the reaction temperature between 15-25°C, add dropwise After the completion of the reaction, keep the temperature between 15-25°C for 2 hours, add the reaction liquid to water (426mL, 3P), filter the precipitated solid with suction, wash the filter cake with ethanol, and dry the obtained solid to obtain 169.85g O- Ethyl tert-butyl acetylhydroxamate, the yield is 85.7%.
[0075] (2) Preparation of compound O-tert-butylhydroxylamine hydrochloride
[0076] Add ethyl O-alkyl substituted acetylhydroxamate (169.85g, 0.85...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com