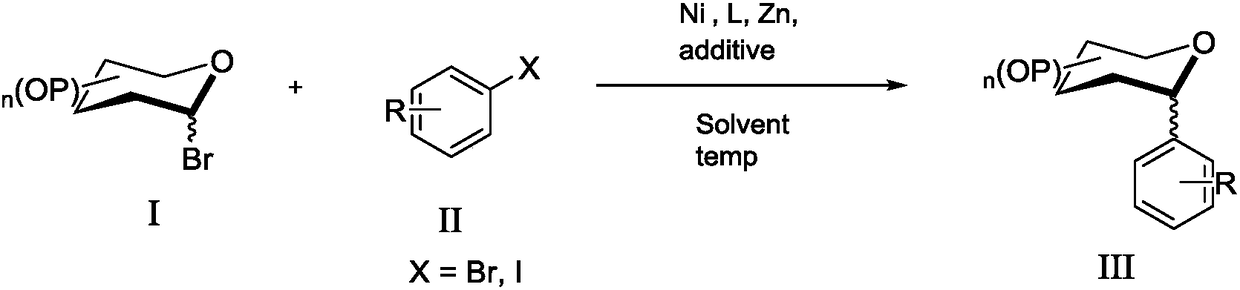
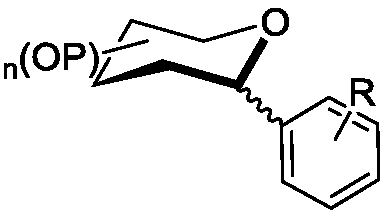
C- aryl glycoside compound and synthesis method thereof
A technology of aryl glycosides and compounds, which is applied in the field of C-aryl glycosides and their synthesis, can solve the problems of poor selectivity of α or β configuration products, complicated operations, cumbersome steps, etc., and achieve cheap catalyst metals and stereoselective Good performance and simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
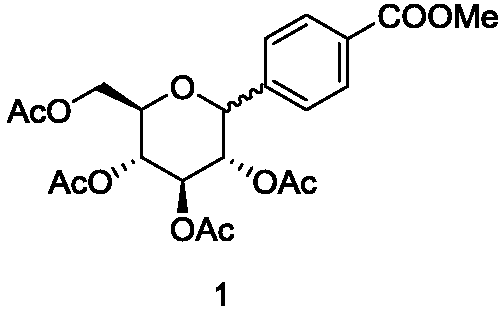
[0054] Example 1: Using Scheme 1, 2,3,4,6-tetraacetoxy-alpha-D-glucopyranose bromide and methyl p-iodobenzoate were used as raw materials to synthesize Product 1, whose structural formula is:
[0055]
[0056] 2,3,4,6-Tetraacetoxy-alpha-D-glucopyranose bromide (0.17mmol, 0.0744g, 120%), methyl p-iodobenzoate (0.15mmol , 0.0395g, 100mol%) zinc powder (0.45mmol, 0.0300g, 300%), anhydrous magnesium chloride (0.15mmol, 0.0143g, 100%), Ni(ClO 4 ) 2 ο6H 2 O (0.03mmol, 0.0110g, 20%), 4-dimethylaminopyridine (0.12mmol, 0.0148g, 80%) and then the Schlenk tube was evacuated three times using a double row tube to ensure the reaction was under nitrogen atmosphere, and finally with a syringe Add DMA(HBr / AcOH):THF=0.2:0.3ml, stir in ice bath for 8-12h. After the reaction, there is no need for post-treatment, and the white solid 1 can be obtained directly by column chromatography (ethyl acetate:petroleum ether=2:8) with a yield of 80-86%. The ratio of type to product is 6:1. α-config...
Embodiment 2
[0065] Example 2: Using 2,3,4,6-tetraacetoxy-alpha-D-glucopyranose bromide and p-iodoanisole as raw materials to synthesize product 2, its structural formula is:
[0066]
[0067] Add 2,3,4,6-tetraacetoxy-alpha-D-glucopyranose bromide (0.225mmol, 0.0744g, 150%), p-iodoanisole (0.15mmol, 0.0330g, 100mol%) zinc powder (0.3mmol, 0.0200g, 200%), anhydrous magnesium chloride (0.075mmol, 0.0074g, 100%), Ni(ClO 4 ) 2 ο6H 2 O (0.015mmol, 0.0055g, 10%), 4,4-di-tert-butyl 2,2-bipyridine (0.0225mmol, 0.0060g, 15%) and then the Schlenk tube was evacuated three times to ensure the reaction Under a nitrogen atmosphere, finally add 1ml of DMA with a syringe, and stir for 8-12h under an ice-water bath. After the reaction, there is no need for post-treatment, and the white solid 2 can be obtained directly by column chromatography (ethyl acetate:petroleum ether=2:8) with a yield of 90-98%. The ratio of type to product is 2:1.
[0068] β-configuration product: 1 H NMR (600MHz, CDCl 3...
Embodiment 3
[0074] Using scheme two, with 2,3,4,6-tetraacetoxy-alpha-D-glucopyranose bromide and 2-(4-fluorophenyl)-5-[(5-iodo-2-methyl Phenyl) methyl] thiophene is raw material synthetic product 3, and its structural formula is:
[0075]
[0076] To a dry Schlenk tube was added sequentially 2,3,4,6-tetraacetoxy-alpha-D-glucopyranose bromide (0.225 mmol, 0.0744 g, 150%), 2-(4-fluorophenyl) -5-[(5-iodo-2-methylphenyl)methyl]thiophene (0.15mmol, 0.0615g, 100mol%), zinc powder (0.3mmol, 0.0200g, 200%), anhydrous magnesium chloride (0.075mmol ,0.0074g,100%),Ni(ClO 4 ) 2 ο6H 2 O (0.015mmol, 0.0055g, 10%), 4,4-di-tert-butyl 2,2-bipyridine (0.0225mmol, 0.0060g, 15%) and then the Schlenk tube was evacuated three times to ensure the reaction Under nitrogen atmosphere, finally add 1ml of DMA with a syringe, and stir for 8-12h under ice-water bath. After the reaction, there is no need for post-treatment, and the white solid 3 can be obtained directly by column chromatography (ethyl acetate:p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com