Bionic nanometer erythrocyte genophore and preparation method and application thereof
A gene carrier and biomimetic nanotechnology, which is applied in the field of biomedical engineering materials, can solve the problems of lack of gene carriers, etc., and achieve the effects of easy-to-obtain raw materials, good biocompatibility, and simple material components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Preparation of charge-reversal cationized bovine serum albumin (cBSA)
[0075] Weigh 50 mg of bovine serum albumin (BSA) and dissolve it in 5 mL of water with pH=4.75 to obtain A solution. Weigh 3.6 mg of EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) and dissolve it in 2 mL of water with pH=4.75, add it dropwise to solution A to obtain solution B . Weigh 2.17 mg DIPEA (N'N-diisopropylethylenediamine) and dissolve it in 2 mL of water with pH=4.75, add it dropwise to solution B, and react at 25°C for 2 hours to obtain solution C. Weigh 70 mg of EDC and dissolve it in 2 mL of water with pH=4.75, and add it dropwise into solution C to obtain solution D. Weighed 3.6 mg PEI600 and dissolved it in 2 mL of water with pH=4.75, added it dropwise to solution D under an ice-water bath, and reacted at 25° C. for 2 hours to obtain solution E. The reaction was stopped by adding acetate buffer. The solution was ultrafiltered four times, and unreacted E...
Embodiment 2
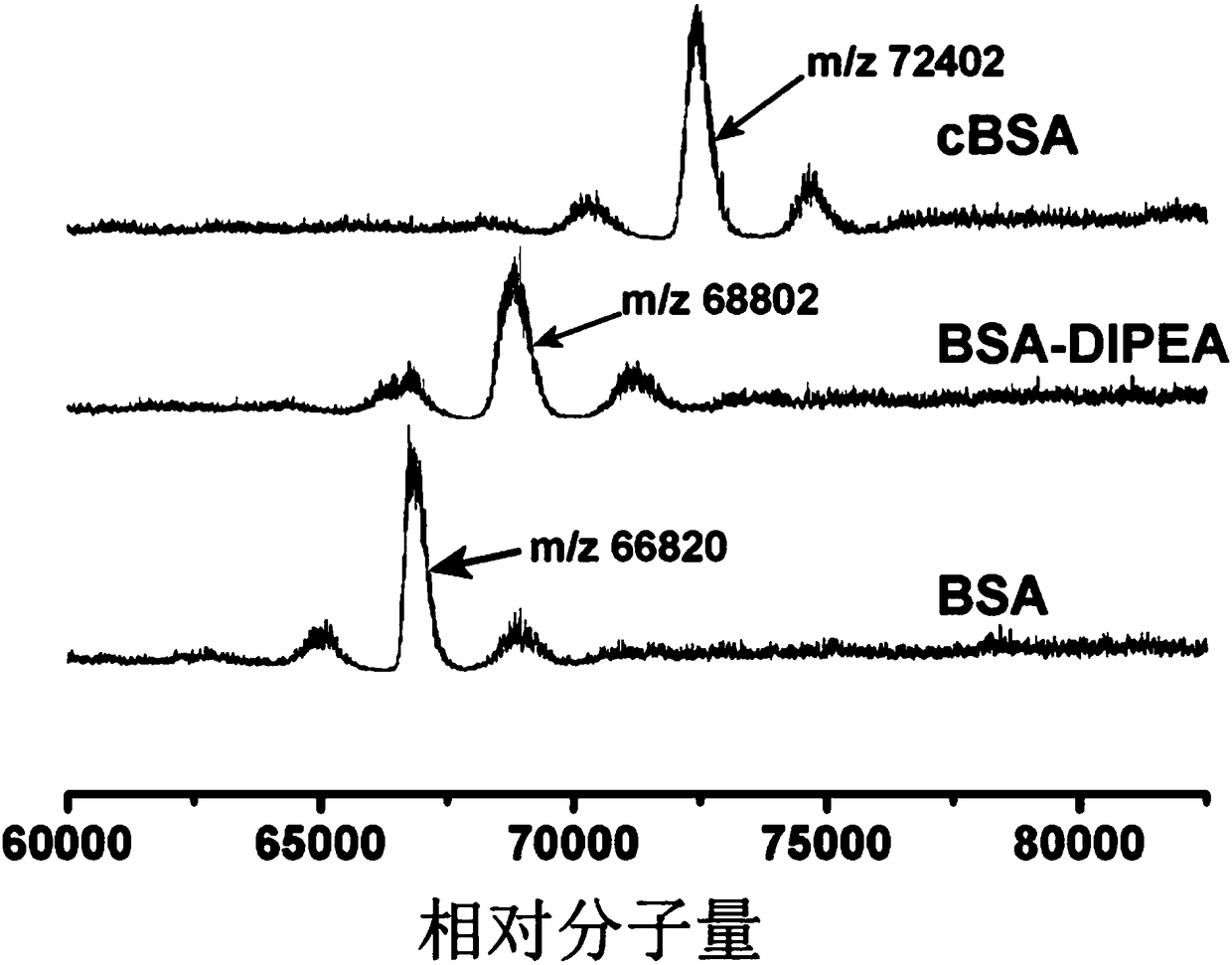
[0077] High-resolution mass spectrometry characterization of embodiment 2 cationized bovine serum albumin (cBSA)
[0078] The cationized bovine serum albumin grafted with different cationic units obtained in Example 1 was dissolved in ultrapure water (1 mg / mL) for high-resolution mass spectrometry characterization. Such as figure 1As shown, the maximum peak value of BSA is 66820Da; the maximum peak value of BSA grafted only with N'N-diisopropylethylenediamine is 68802Da; The largest peak is 72402Da. figure 1 The results proved that the cationic bovine serum albumin (cBSA) was successfully synthesized in this step reaction.
Embodiment 3
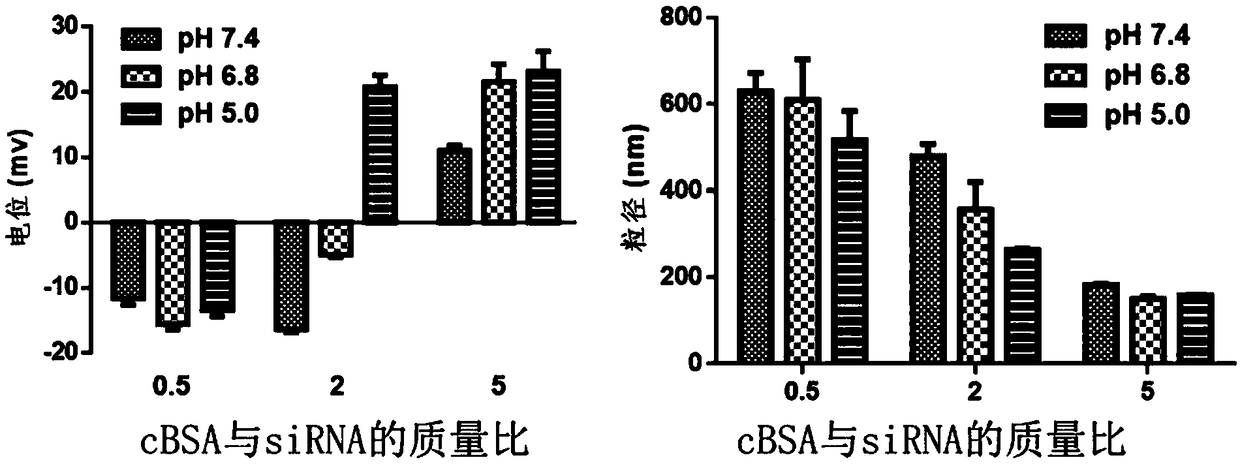
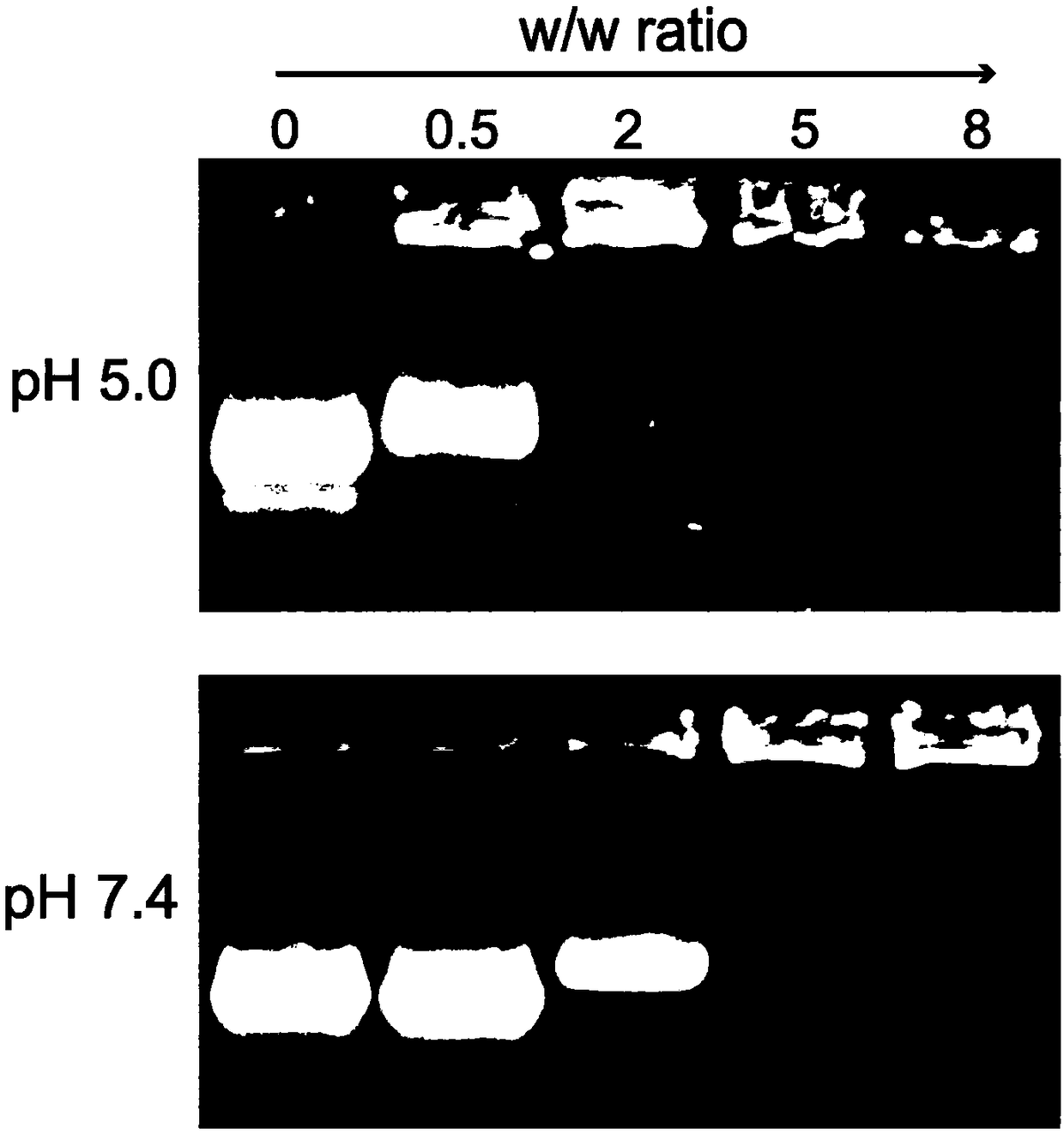
[0079] Example 3 Preparation of cBSA-siRNA nanocomplex
[0080] Weigh 10 mg of cBSA and dissolve it in 10 mL of ultrapure water, and prepare siRNA (VEGF-siRNA, purchased from Suzhou Gemma Gene Co., Ltd.), whose sequence is 5'-GGAUCAAACCUCACCAAAGTTCUUUGGUGAGGUUUGAUCCTT-3' (SEQ ID NO.1) into 1 mg / mL of stock solution. The cBSA-siRNA nanocomplexes were prepared under the environment of pH 5 or 7.4 respectively. According to a series of mass ratios set, the cBSA solution was quickly added to the siRNA stock solution, and incubated at 25°C for 30 minutes to obtain a series of Nanocomposites with different surface charge properties in a neutral environment, wherein the mass ratios of cBSA to siRNA are 0.5, 2, 5, and 8, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com