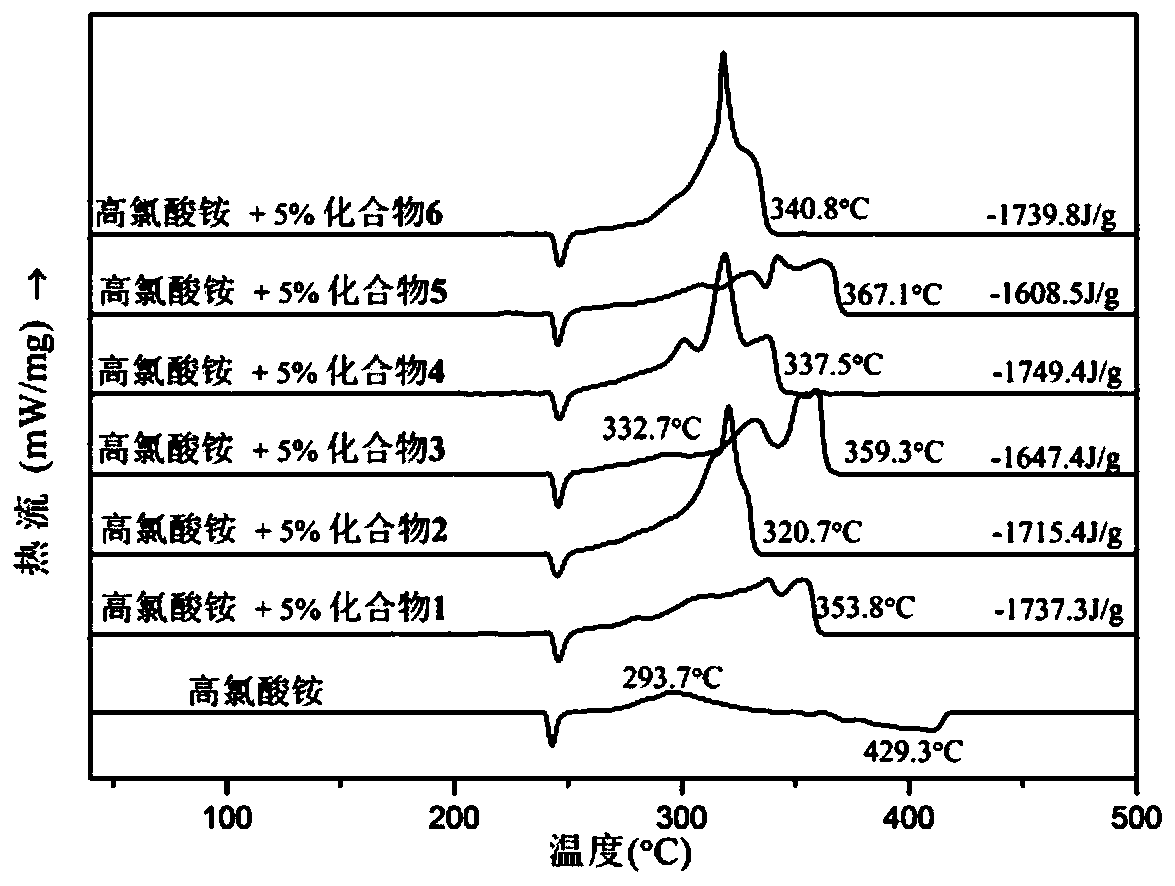
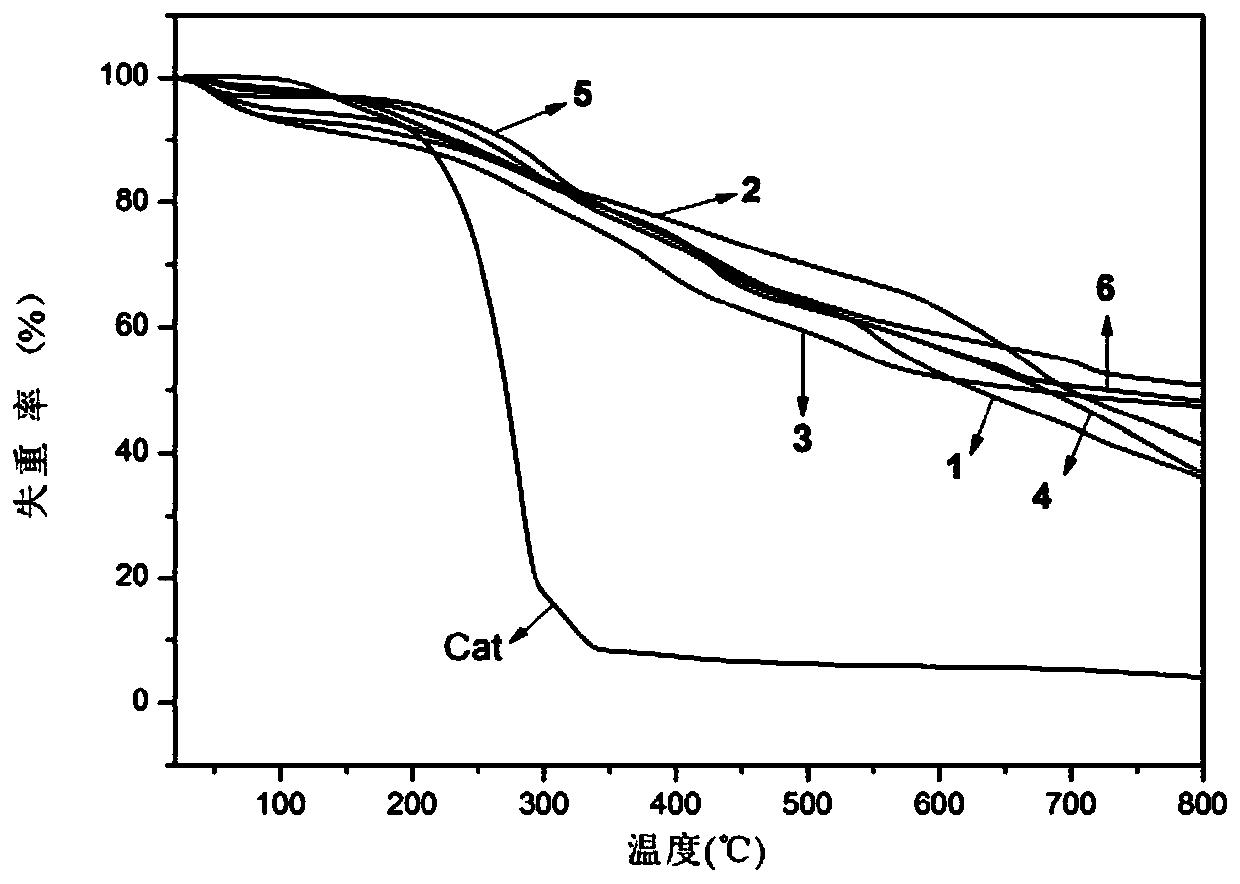
High nitrogen dinuclear ferrocene triazole ionic metal complex and preparation method thereof
A technology for nuclear ferrocenetriazole and metal complexes, applied in the field of binuclear ferrocenetriazole ionic metal complexes and their preparation, achieving high yield, excellent catalytic performance, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
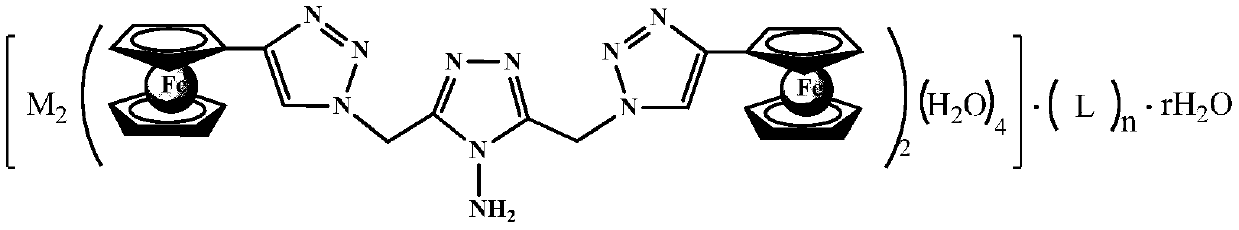
[0020] 202mg (0.5mmol) Fe(NO 3 ) 3 9H 2O was completely dissolved in a 50mL round bottom flask filled with 5mL of distilled water. At 70°C, 10mL of methanol solution containing 270g (1.5mmol) of potassium 1,1,3,3-tetracyanoacrylate and 5mL of 316mg (0.5mmol) N,N of 4-amino-3,5-bis(4-ferrocenyl-1,2,3-triazole-1-methyl)-1,2,4-triazole -Dimethylformamide solution, react at constant temperature for 3 hours after the dropwise addition, filter with suction, wash the filter cake twice with N,N-dimethylformamide, then fully wash with distilled water and methanol in turn, and then dry in vacuum Dry at 70° C. for 24 hours in an oven to obtain a high-nitrogen dinuclear ferrocenetriazolium ionic metal complex (referred to as compound 1) with the following structural formula, with a yield of 77%.
[0021]
[0022] The structural characterization data of the obtained complex is: FT-IR (KBr, cm -1 ):3131s, 2955m, 2206s, 1789s, 1711s, 1638s, 1600m, 1542s, 1509s, 1463m, 1378s, 1333s, 12...
Embodiment 2
[0024] In this example, equimolar Cu(NO 3 ) 2 ·3H 2 O replaces Fe(NO in Example 1 3 ) 3 9H 2 O, 1,1,3,3-tetracyanoacrylate potassium consumption is 180g (1.0mmol), and other steps are identical with embodiment 1, obtain the high nitrogen dinuclear ferrocene triazole ionic metal complex ( Denoted as compound 2), the yield was 71%.
[0025]
[0026] The structural characterization data of the obtained complex is: FT-IR (KBr, cm -1 ):3131s, 3001m, 2199s, 1782s, 1704s, 1633s, 1548s, 1502s, 1437m, 1327s, 1197m, 1105s, 1047s, 871s, 818s, 662m; EA: C 84 Cu 2 h 72 Fe 4 N 36 o 6 Theoretical value C 49.65%, H 3.57%, N 24.81%, measured value C 49.68%, H 3.79%, N 24.92%; UV-Vis: 263nm (ε=3.29×10 5 ), 397nm (ε=1.31×10 5 ), 417nm (ε=1.24×10 5 ).
Embodiment 3
[0028] In this example, the potassium 1,1,3,3-tetracyanoacrylate in Example 1 is replaced with equimolar 1,1,2,3,3-pyridine pentacyanoacrylate, other steps are the same as in Example 1 Similarly, a high-nitrogen dinuclear ferrocenetriazole ionic metal complex (referred to as compound 3) with the following structural formula was obtained with a yield of 71%.
[0029]
[0030] The structural characterization data of the obtained complex is: FT-IR (KBr, cm -1 ):3203s, 2949m, 2199s, 1770s, 1698s, 1624s, 1502s, 1431s, 1378vs, 1333s, 1223s, 1105m, 1053s, 962s, 877s, 818s; EA: C 104 h 84 Cr 2 Fe 4 N 50 o 14 Theoretical value C 48.31%, H 3.27%, N 27.09%, measured value C 48.41%, H 3.27%, N 27.23%; UV-Vis: 264nm (ε=3.13×10 5 ), 395nm (ε=1.05×10 5 ), 416nm (ε=9.44×10 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat release | aaaaa | aaaaa |
| heat release | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com