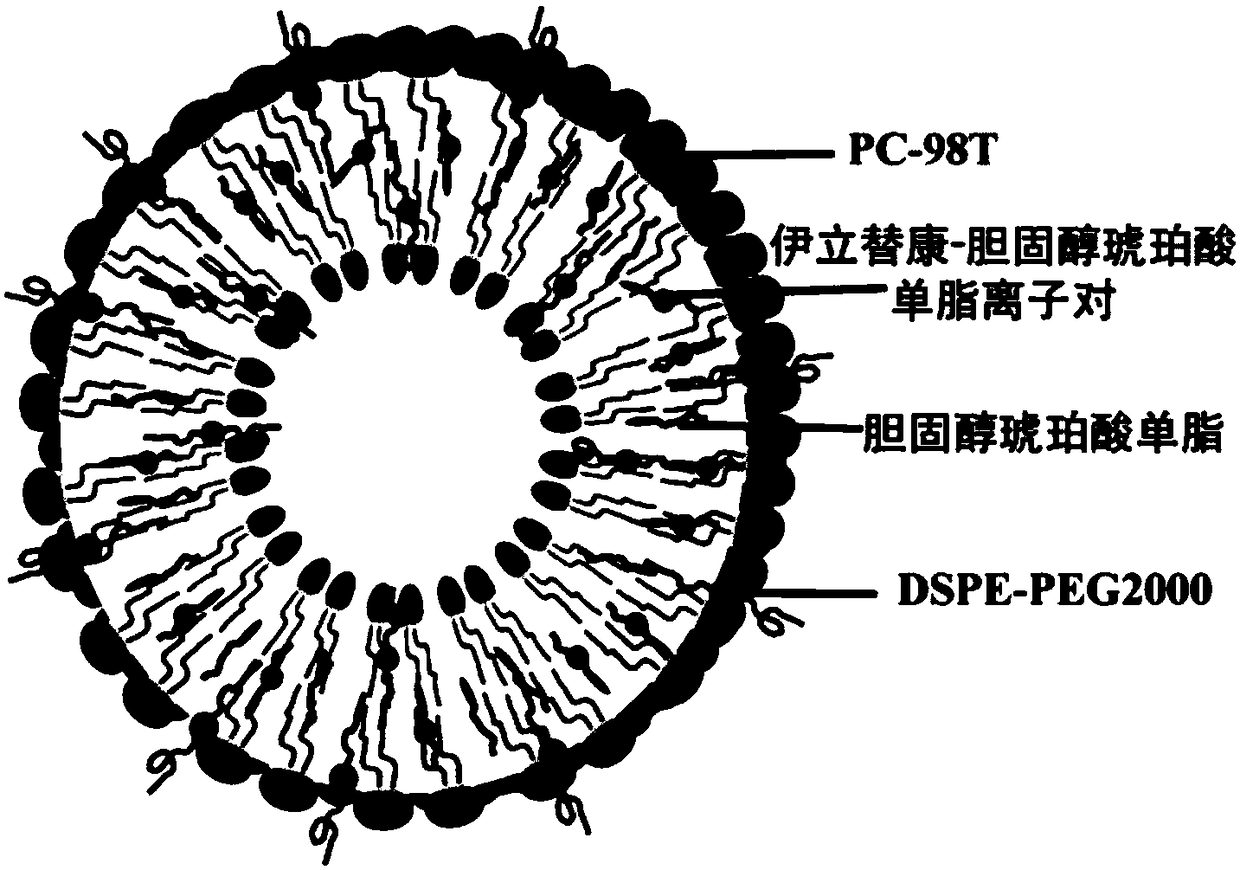
Irinotecan-cholesterol succinic acid simple lipid ion pair, liposome and preparation method and application thereof
A technology of cholesterol succinic acid monoester and succinic acid monoester, which is applied in the field of irinotecan-cholesterol succinic acid monoester ion pair and liposome and preparation, which can solve problems such as instability, low encapsulation efficiency, and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
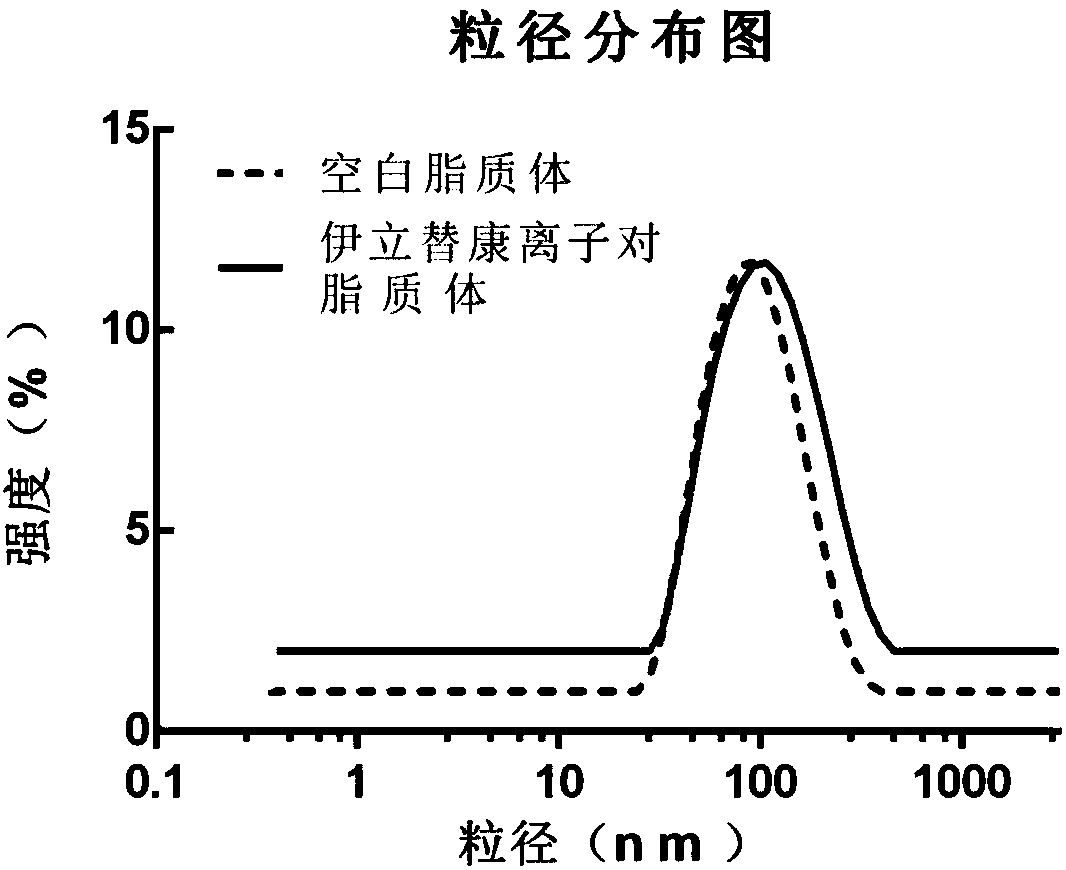
Image
Examples
Embodiment 1
[0046] Preparation of ion pair of irinotecan-cholesterol succinate monoester
[0047] Weigh irinotecan hydrochloride, add appropriate amount of ultrapure water, stir to form a slurry, add 1mol / L NaHCO drop by drop 3 Solution, wherein, the irinotecan hydrochloride and NaHCO that take by weighing are equimolar ratio; Continue to stir until reaction is complete. Centrifuge, discard the supernatant, wash the precipitate with ultrapure water 2 to 3 times, and collect the precipitate. Vacuum dry at 40°C for 2h. The finally obtained off-white powder is irinotecan free base, which is stored in a vial and kept in a refrigerator at 4°C in the dark for future use. Add an appropriate amount of chloroform solution to dissolve irinotecan free base and cholesterol succinate monoester in an equimolar ratio, stir in an ice bath for 1.5 hours, add the anti-solvent methyl tert-butyl ether drop by drop, the solution becomes cloudy and stops, and place it in a fume hood The solvent was evaporat...
Embodiment 2
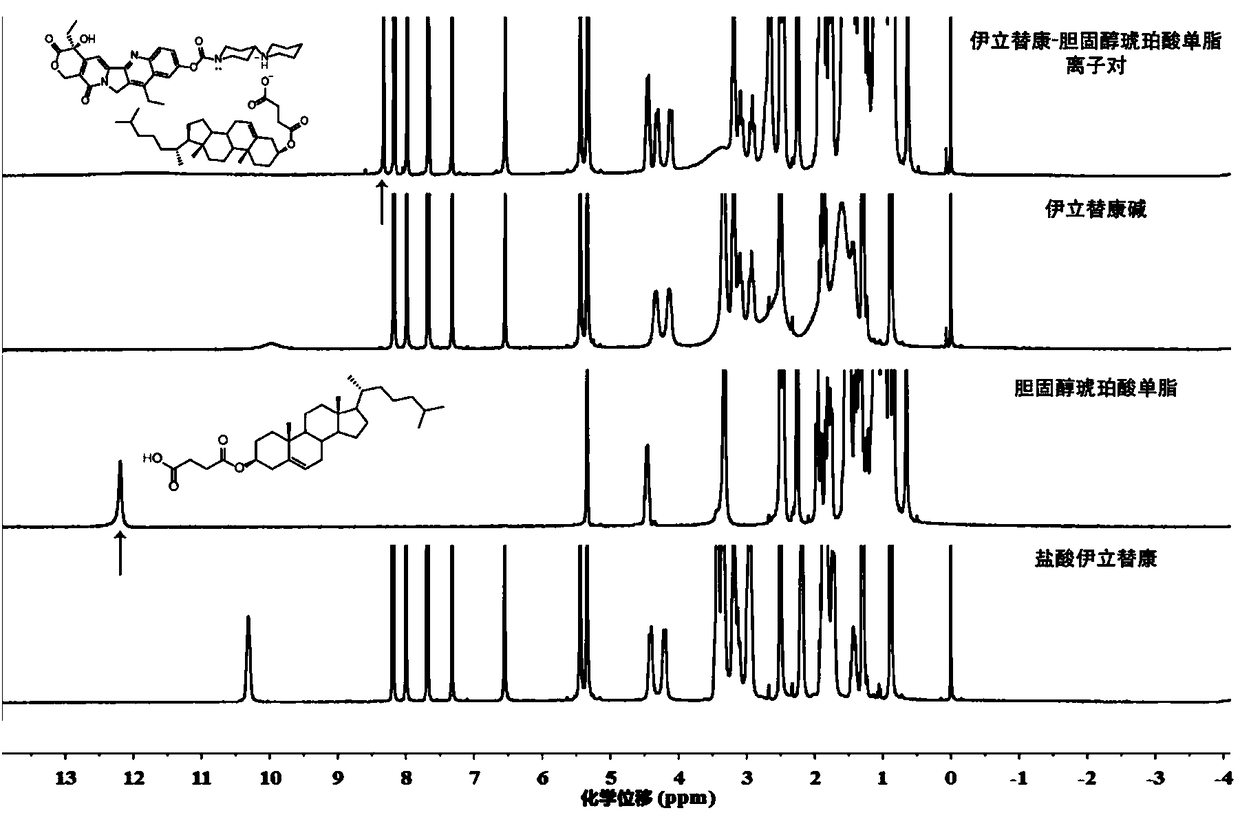
[0049] Hydrogen-NMR Spectroscopy Research
[0050] Weigh about 5 mg of samples of irinotecan hydrochloride, irinotecan base, cholesterol succinate monoester and irinotecan-cholesterol succinate monoester ion pair, add an appropriate amount of DMSO-d6 to dissolve, and ultrasonicate for 5 minutes until the solution is completely clear , transferred to an NMR sample tube, sealed, and allowed to stand at 20°C for a period of time. Bruker Advance-400MHz equipment was used for detection, and TMS was used as an internal standard substance for chemical shift (δ) determination. Measurement results such as figure 2 As shown, the occurrence of hydrogen proton transfer will be observed more intuitively. In the NMR spectrum of irinotecan base, there is no active hydrogen in the low field region. In the downfield region of cholesterol succinate monoester and irinotecan-cholesterol succinate monoester ion pair, there are obvious active hydrogen peaks. The hydroxyl hydrogen of cholestero...
Embodiment 3
[0052] Determination of Solubility and Apparent Oil-Water Partition Coefficient
[0053] According to Chinese Pharmacopoeia (2010 edition) two appendix solubility determination method, take excessive irinotecan hydrochloride crude drug powder, irinotecan free base powder, irinotecan-cholesterol succinic acid monoglyceride ion pair powder is placed in water for injection respectively , and then placed in a constant temperature oscillator at 37°C, shaken at 100rpm for 72h, after standing at room temperature for a period of time, centrifuge to take the supernatant, filter it with a 0.22μm microporous membrane, take the subsequent filtrate and dilute it properly, and measure its drug concentration by HPLC , and the solubility results of each saturated solution are shown in Table 1.
[0054] Table 1 Solubility of irinotecan and irinotecan-cholesterol succinate monoester ion pair
[0055]
[0056] According to the provisions of the Chinese Pharmacopoeia, the oil-water partition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com