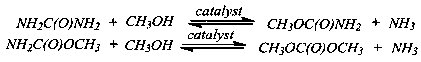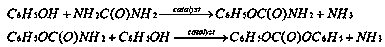Preparation method and application of core-shell catalyst which adopts transition metal salt as core and nano-molecular sieve as shell
A technology of nano-molecular sieves and transition metal salts, which is applied in the direction of molecular sieve catalysts, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as difficult separation, achieve easy-to-obtain raw materials, simple preparation process, and increase yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: In this example, the core-shell catalyst with transition metal salt as the core silicalite-1 molecular sieve as the shell is ZnCl 2 @silicallite-1 core-shell catalyst;
[0033] A method for preparing a core-shell catalyst with a transition metal salt as the core silicalite-1 molecular sieve as the shell, and the specific steps are as follows:
[0034] (1) Preparation of monodisperse spherical zinc oxide powder;
[0035] (2) The spherical zinc oxide powder of step (1) is immersed in the negative polyelectrolyte solution for 10 minutes, and centrifuged to obtain filtrate I and filter residue I; the solid-to-liquid ratio of the spherical zinc oxide powder to the negative polyelectrolyte solution is g: mL is 0.1:50; the mass percentage concentration of the negative polyelectrolyte in the negative polyelectrolyte solution is 0.3%, and the negative polyelectrolyte is poly(p-styrenesulfonic acid) PPS;
[0036] (3) The filter residue I of step (2) is immersed in the positive...
Embodiment 2
[0045] Example 2: In this example, the core-shell catalyst with transition metal salt as the core silicalite-1 molecular sieve as the shell is CuBr 2 @silicallite-1 core-shell catalyst;
[0046] A method for preparing a core-shell catalyst with a transition metal salt as the core silicalite-1 molecular sieve as the shell, and the specific steps are as follows:
[0047] (1) Preparation of monodisperse spherical copper oxide powder;
[0048] (2) The spherical copper oxide powder of step (1) was immersed in the negative polyelectrolyte solution for 15 minutes, and centrifuged to obtain filtrate I and filter residue I; the solid-to-liquid ratio of the spherical copper oxide powder to the negative polyelectrolyte solution g: mL is 1:60; the mass percentage concentration of the negative polyelectrolyte in the negative polyelectrolyte solution is 5.0%, and the negative polyelectrolyte is poly(p-styrene sulfonic acid) PPS;
[0049] (3) Immerse the filter residue I of step (2) in the positive ...
Embodiment 3
[0058] Example 3: In this example, the core-shell catalyst with transition metal salt as the core silicalite-1 molecular sieve as the shell is Ni(NO 3 ) 2 @silicallite-1 core-shell catalyst;
[0059] A method for preparing a core-shell catalyst with a transition metal salt as the core silicalite-1 molecular sieve as the shell, and the specific steps are as follows:
[0060] (1) Preparation of monodisperse spherical nickel hydroxide powder;
[0061] (2) The spherical nickel hydroxide powder of step (1) is immersed in the positive polyelectrolyte solution for 20 minutes, and centrifuged to obtain filtrate I and filter residue I; the solid-to-liquid ratio of the spherical nickel oxide powder to the negative polyelectrolyte solution is g :mL is 0.5:55; the mass percentage concentration of the negative polyelectrolyte in the negative polyelectrolyte solution is 2.45%, and the negative polyelectrolyte is poly(p-styrene sulfonic acid) PPS;
[0062] (3) The filter residue I of step (2) is imm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com