Oxygen group cobalt-based catalyst, preparation method and application of electrocatalytic oxygen evolution
A catalyst and electrocatalyst technology, which is applied in the directions of cobalt oxide/cobalt hydroxide, metal selenide/telluride, electrodes, etc. Due to the problems of poor reproducibility of the base catalyst, it can achieve the effect of good oxygen evolution catalytic activity, good electrochemical oxygen evolution performance and controllable morphology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
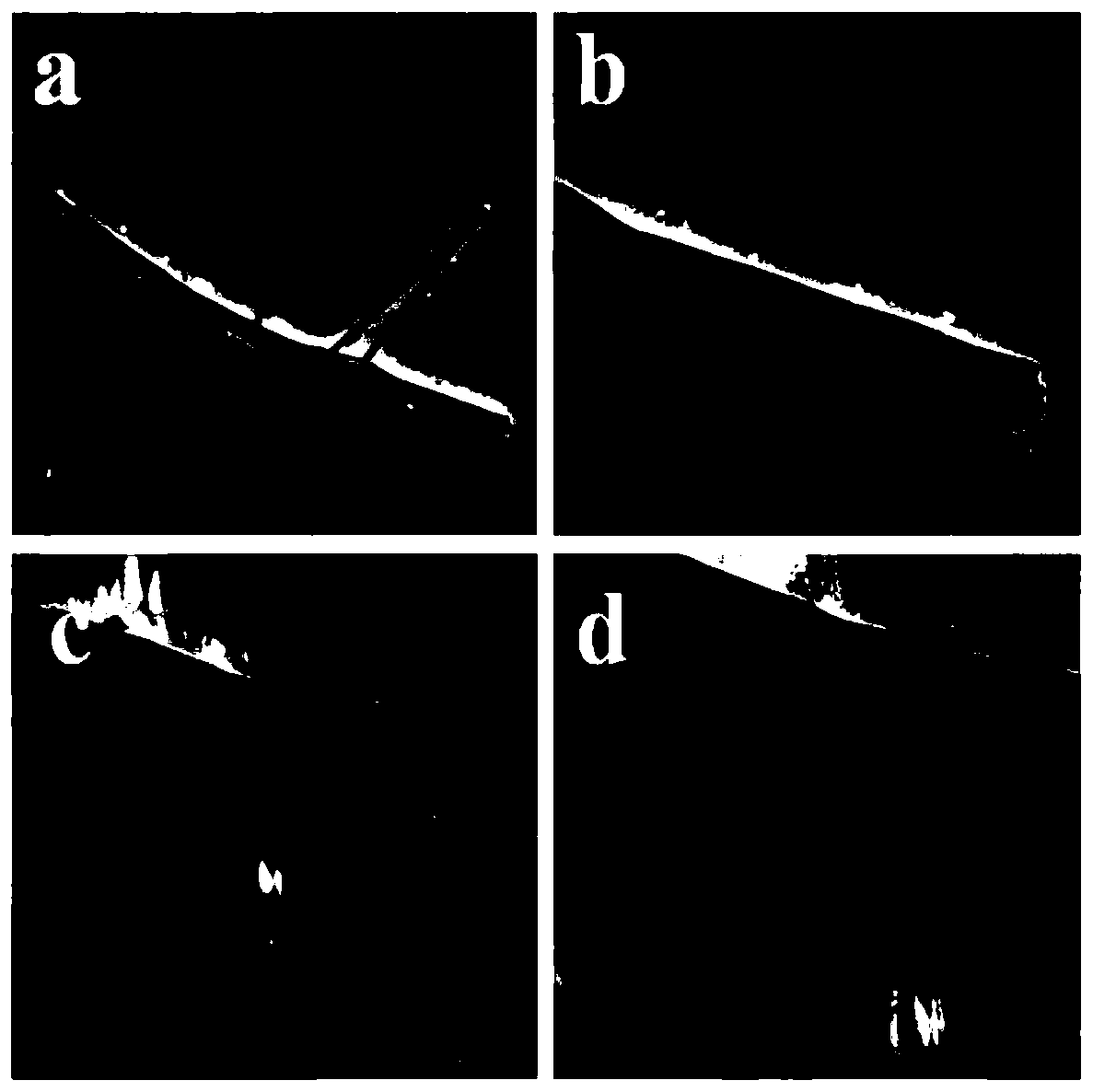
[0036] Dissolve 100mg of cobalt nitrate hexahydrate and 30mg of urea in secondary water, and ultrasonicate for a period of time to form a uniform solution. Transfer to a reactor and react at 180°C for 1h. After the reaction is completed, cool to room temperature, centrifuge, and wash the solid powder with ethanol several times to separate organic impurities and by-products in the product. Then put the product into a vacuum oven and dry at room temperature for 12 hours. A fuchsia precursor was obtained. The morphology of the prepared cobalt tetroxide precursor was characterized by transmission electron microscopy, as shown in figure 1a, 1b shown.
[0037] The prepared precursor was put into a tube furnace and reacted at 300 °C for 2 h in an air atmosphere. Wash the obtained product once with water and ethanol, then put it into a vacuum drying oven, and dry it at room temperature for 12 hours to obtain rod-shaped tricobalt tetroxide, which is the cobalt-based oxygen evolutio...
Embodiment 2
[0041] Dissolve 100mg of cobalt nitrate hexahydrate and 30mg of urea in secondary water, and ultrasonicate for a period of time to form a uniform solution. Transfer to the reaction kettle and react at 180°C for 11h. After the reaction is completed, cool to room temperature, centrifuge, and wash the solid powder with ethanol several times to separate organic impurities and by-products in the product. Then put the product into a vacuum oven and dry at room temperature for 12 hours. A fuchsia precursor was obtained. The morphology of the prepared flaky cobalt trioxide precursor was characterized by transmission electron microscopy, as shown in figure 1 c, 1d shown.
[0042] The prepared flake precursor was put into a tube furnace and reacted at 300 °C for 2 h in an air atmosphere. Wash the obtained product once with water and ethanol, then put it into a vacuum drying oven, and dry it at room temperature for 12 hours to obtain flaky cobalt tetroxide, which is the cobalt-based ...
Embodiment 3
[0046] Put 10 mg of selenium powder into a mixed solution of 3 mL of ethylene glycol and 1 mL of hydrazine, ultrasonicate for a period of time, add 5 mg of rod-shaped cobalt trioxide nanopowder prepared in Example 1, continue ultrasonication, transfer to a reaction kettle, and react at 180°C for 12 hours. After the reaction is completed, cool to room temperature, wash with ethanol several times, and dry at room temperature in vacuum for one day to obtain rod-shaped cobalt selenide, which is the cobalt-based oxygen evolution catalyst of the present invention.
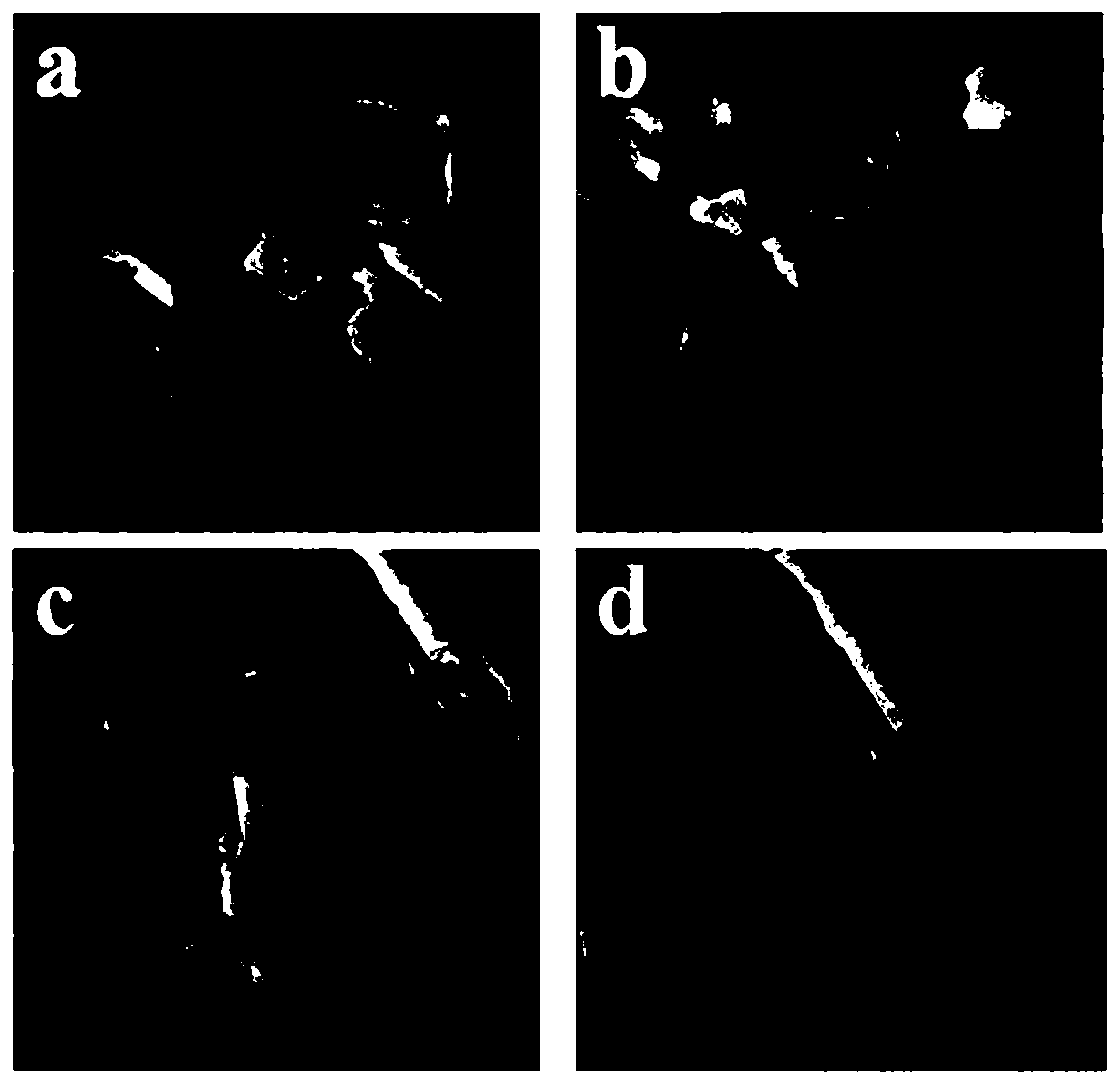
[0047] The prepared cobalt selenide was characterized by transmission electron microscopy, as shown in image 3 a, 3b shown. It can be seen that the morphology of the catalyst is nanorod.
[0048] Test the oxygen evolution performance of the cobalt-based oxygen evolution catalyst prepared in this embodiment, such as Figure 4 shown. Depend on Figure 4 It can be seen that the current density is 50mV / cm 2 , the overp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com