Application of CuO-CuCo2O4 catalyst in electrochemical CO2 reduction
A cuo-cuco2o4, 1.cuo-cuco2o4 technology is applied in the application field of CuO-CuCo2O4 catalyst in electrochemical reduction of CO2, achieving the effects of excellent electrochemical performance, broad application prospects and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Solvothermal Synthesis of CuO-CuCo in Isopropanol / Glycerol Mixed Solvent System 2 o 4 The preparation method of catalyst comprises the following steps:
[0038] Step 1: Weigh 0.107g of cobalt nitrate trihydrate and 0.072g of copper nitrate trihydrate into a 100mL beaker, then add 36mL of isopropanol, then add 18mL of glycerin, and magnetically stir until the reactants are completely dissolved;
[0039] The second step: transfer the solution obtained in the first step to a 100mL hydrothermal kettle, and react at a constant temperature of 180°C for 12h;
[0040] Step 3: Cool the reaction product of the second step to room temperature, then centrifuge, wash, and dry;
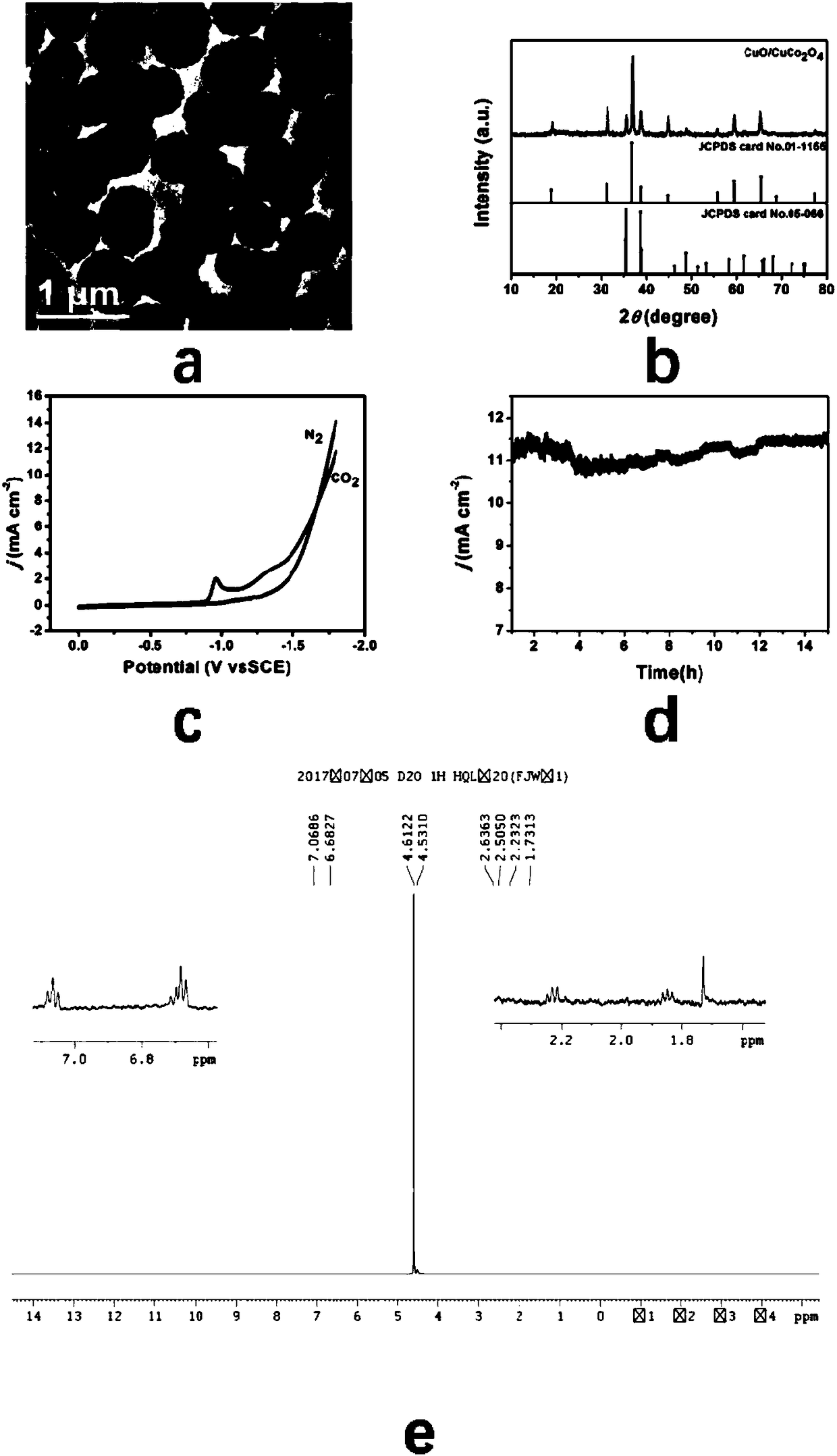
[0041]Step 4: Take out 50 mg of the dried product in the third step and put it in a quartz magnetic boat, and then calcinate at a constant temperature of 600 °C for 2 hours at a heating rate of 1 °C / min in an air atmosphere to obtain a hollow structure CuO-CuCo 2 o 4 catalyst.
[0042] Prepared CuO-CuCo...
Embodiment 2
[0044] Solvothermal Synthesis of CuO-CuCo in Isopropanol / Ethylene Glycol Mixed Solvent System 2 o 4 The preparation method of catalyst comprises the following steps:
[0045] Step 1: Weigh 0.87g of cobalt acetate and 0.051g of copper chloride dihydrate into a 100mL beaker, then add 45mL of isopropanol, then add 5mL of ethylene glycol, and stir magnetically until the reactants are completely dissolved;
[0046] The second step: transfer the solution obtained in the first step to a 100mL hydrothermal kettle, and react at a constant temperature of 180°C for 12h;
[0047] Step 3: Cool the reaction product of the second step to room temperature, then centrifuge, wash, and dry;
[0048] The fourth step: take out 50 mg of the dried product in the third step and put it in a quartz magnetic boat, and then calcinate at a constant temperature of 450°C for 2 hours at a heating rate of 2°C / min in an air atmosphere to obtain CuO-CuCo 2 o 4 catalyst.
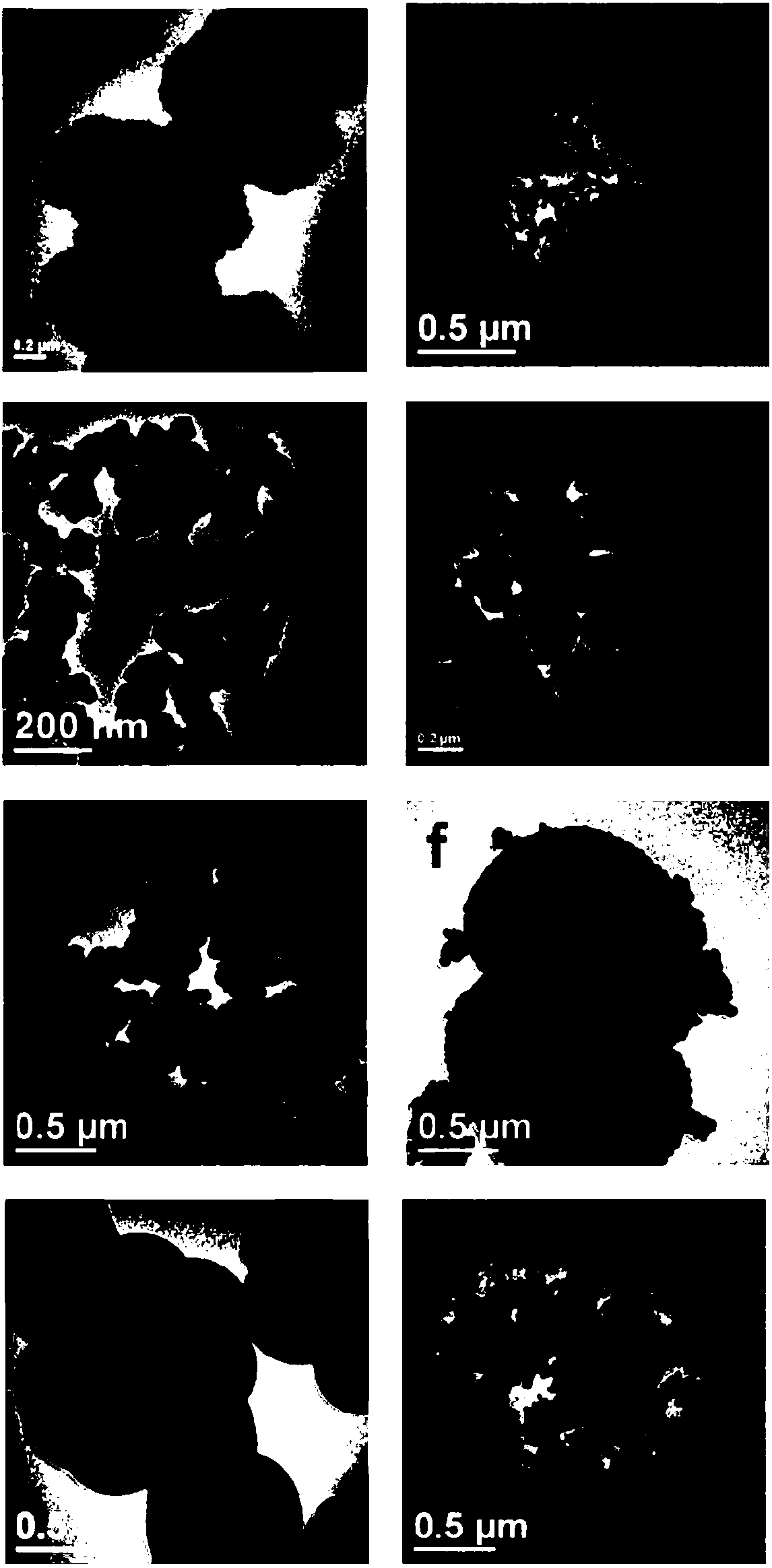
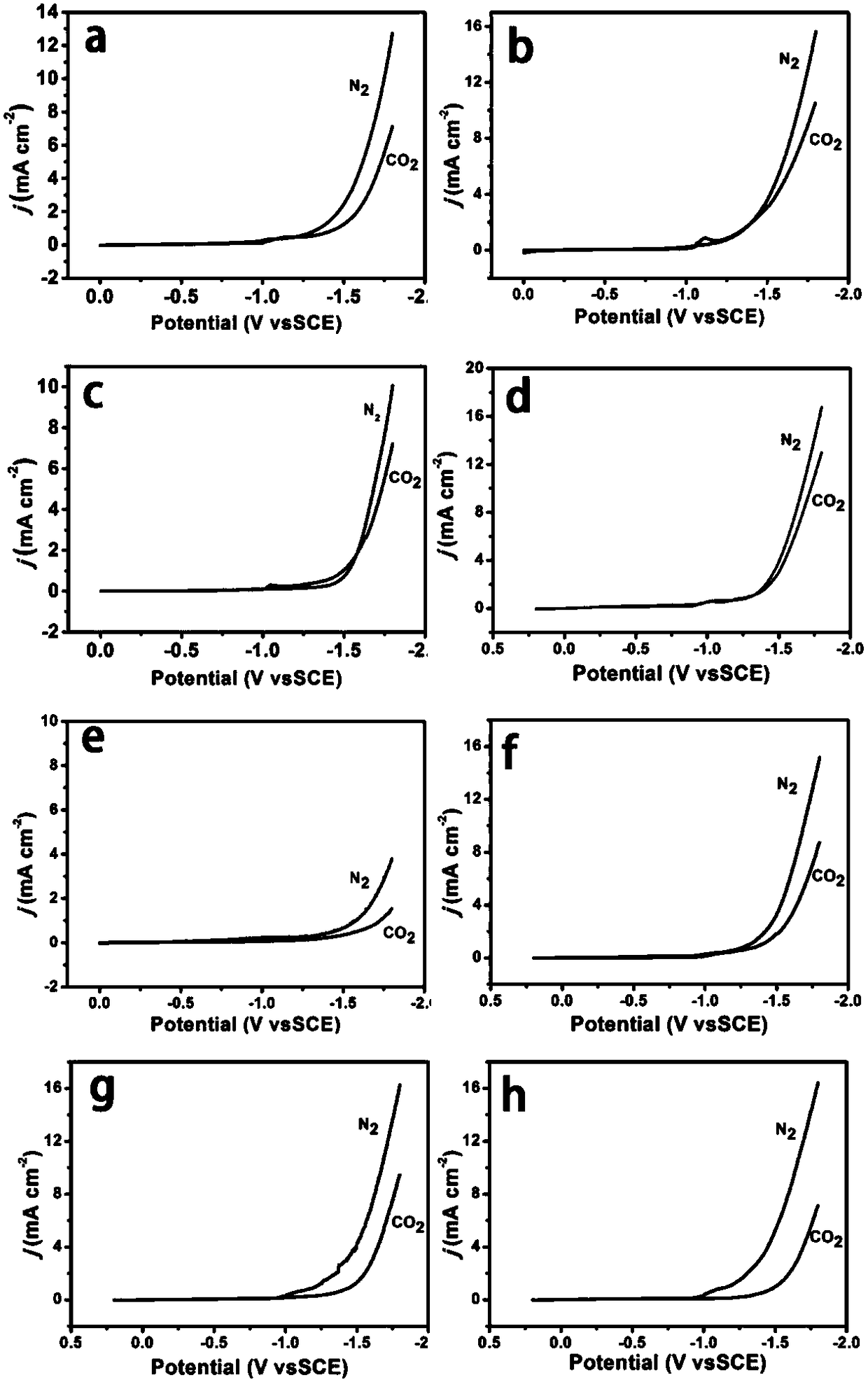
[0049] figure 2 a and image 3 ...
Embodiment 3
[0051] Solvothermal Synthesis of CuO-CuCo in Ethanol / Glycerol Mixed Solvent System 2 o 4 The preparation method of catalyst comprises the following steps:
[0052] Step 1: Weigh 0.087g of cobalt nitrate hexahydrate and 0.048g of copper sulfate into a 100mL beaker, then add 30mL of ethanol, then add 30mL of glycerin, and stir magnetically until the reactants are completely dissolved;
[0053] The second step: transfer the solution obtained in the first step to a 100mL hydrothermal kettle, and react at a constant temperature of 180°C for 12h;
[0054] Step 3: Cool the reaction product of the second step to room temperature, then centrifuge, wash, and dry;
[0055] Step 4: Take out 50 mg of the dried product in the third step and put it in a quartz magnetic boat, and then calcinate at a constant temperature of 400°C for 2 hours at a heating rate of 5°C / min in an air atmosphere to obtain CuO-CuCo 2 o 4 catalyst.
[0056] figure 2 b and image 3 b is the CuO-CuCo prepared i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com