Bacteroides fragilis capable of relieving endotoxin infection as well as application thereof
A technology of Bacteroides fragilis and microbial strains, applied in the field of microbiology, can solve the problems of unconcerned strains, change intestinal permeability, etc., and achieve the effects of low immunogenicity, increased concentration, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Cultivation and preservation of Bacteroides fragilis CCFM1020
[0048] 1. Preparation of culture medium: Prepare brain-heart infusion BHI medium (such as the product of Qingdao Haibo Biotechnology Co., Ltd.), dissolve it in distilled water, and add cysteine hydrochloride 1g / L, hemin 0.01g / L, vitamin K 10.002g / L, mix evenly, then adjust the pH to 7.0, and sterilize at 115-121°C for 15-20min to obtain the culture medium.
[0049] 2. Cultivation method: Bacteroides fragilis CCFM1020 strains are inoculated according to the inoculation amount of 2-4% based on the weight of the above-mentioned culture medium, and anaerobically cultured at 37° C. for 12-18 hours, reaching a stable period.
[0050] 3. Preparation of protective agent: Weigh 1 g / L of cysteine hydrochloride and 200 g / L of glycerin, dissolve them in distilled water evenly, and sterilize at 115-121° C. for 15-20 minutes to obtain the protective agent.
[0051] 4. Preservation method: After cleaning t...
Embodiment 2
[0052] Example 2: Tolerance dose experiment of Bacteroides fragilis CCFM1020 administered to mice
[0053] Get 10 healthy female C57 mice of the age of 6-8 weeks, and the Bacteroides fragilis CCFM1020 cryopreservation agent (10 10 CFU / ml), observed for 5 days, and recorded the body weight and death status of the mice.
[0054] The results are shown in Table 1, and the feeding concentration was 10 9 CFU of Bacteroides fragilis CCFM1020 had no significant effect on the mice, with weight gain and no death. The appearance of the mice had no obvious pathological symptoms.
[0055] Table 1: Feeding 10 9 Effect of CFU of Bacteroides fragilis CCFM1020 on body weight of mice
[0056]
Embodiment 3
[0057] Example 3: Immune tolerance experiment of mice fed with Bacteroides fragilis CCFM1020
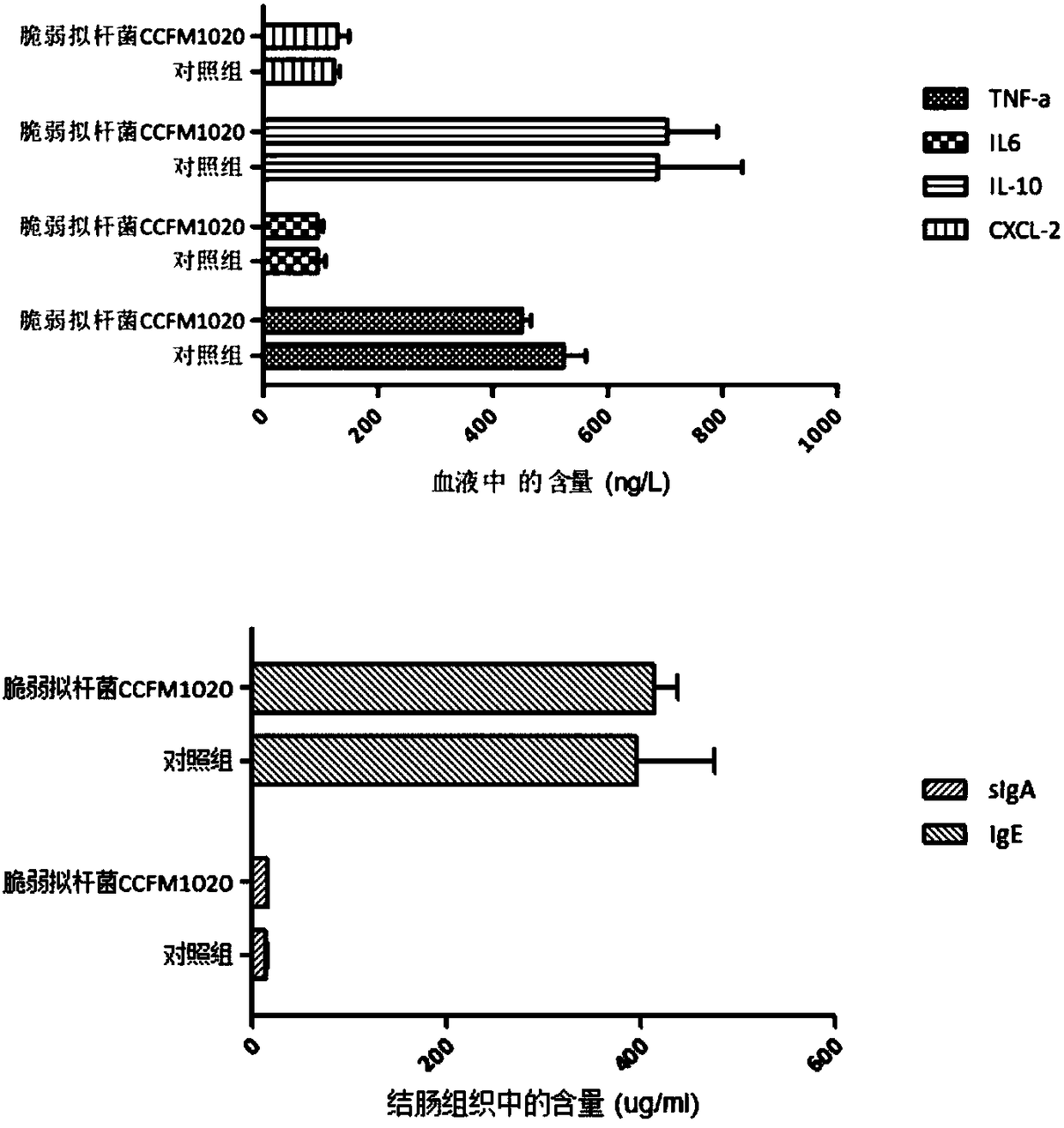
[0058] Twenty healthy female C57 mice aged 6-8 weeks were randomly divided into two groups: the negative control group and the B. fragilis CCFM1020 intervention group, with 10 mice in each group. The mice in the Bacteroides fragilis CCFM1020 intervention group were given 0.1ml of the above-mentioned Bacteroides fragilis CCFM1020 cryopreservation agent once every 24 hours (10 10 CFU / ml), the mice in the control group were fed with 0.1ml of the protective agent of the above-mentioned cryopreservation agent every 24 hours, and all the mice were sacrificed after continuous feeding for 5 days. Technology Co., Ltd.) after lysing and homogenizing, the concentration of immune factors was determined using an ELISA kit (product of Nanjing Senbega Biotechnology Co., Ltd.).
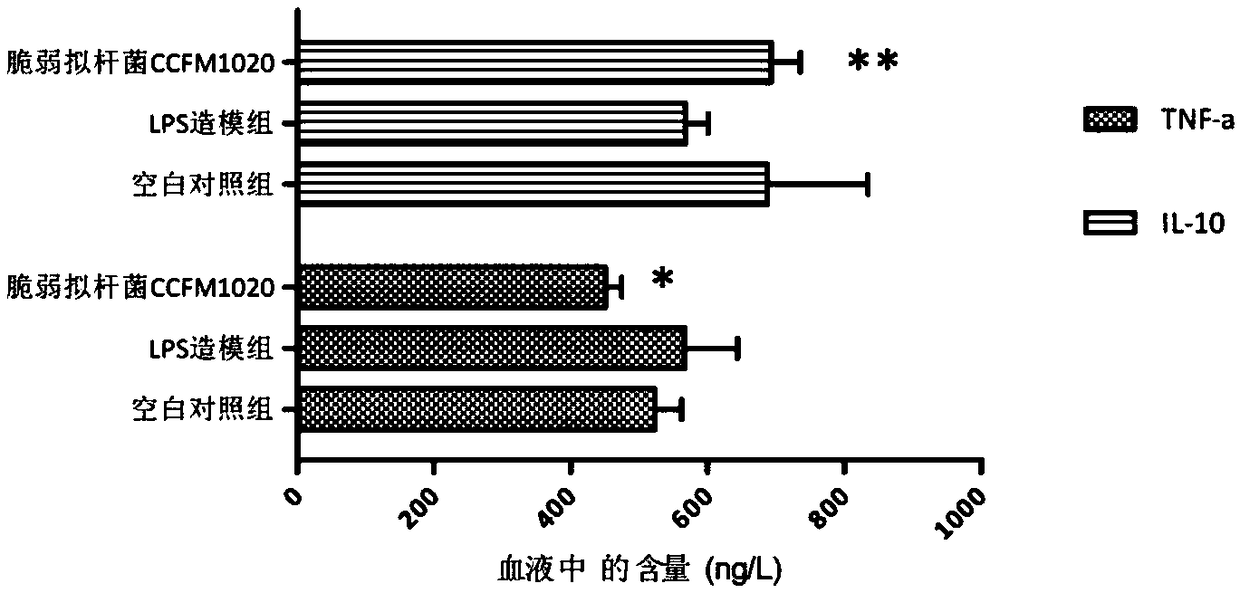
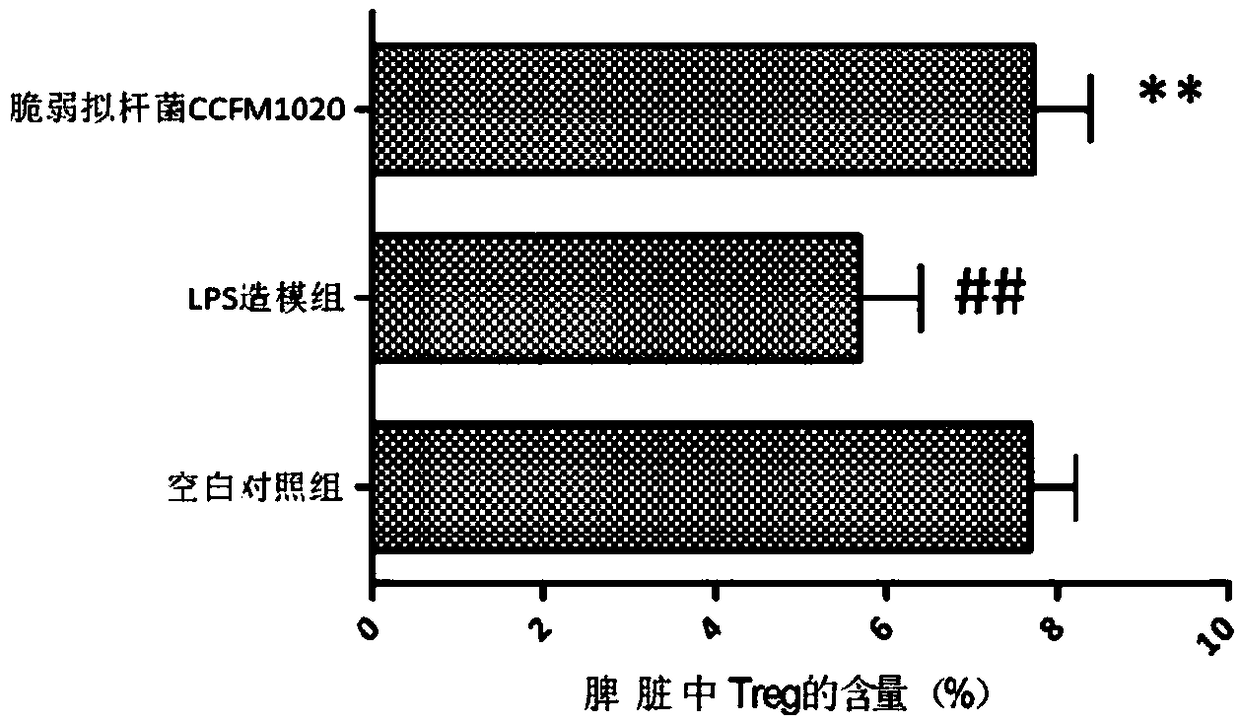
[0059] The experimental results are attached figure 1 As shown, the contents of immune factors TNF-α, IL-6, IL-10 and C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com