Compound as well as application and preparation method thereof to surface activation
A compound and synthesis method technology, applied in the preparation of organic compounds, chemical instruments and methods, and sulfide preparation, can solve the problems of limited physical and chemical properties of surfactants, no obvious improvement in yield, and high reaction costs, etc., to achieve High yield, mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
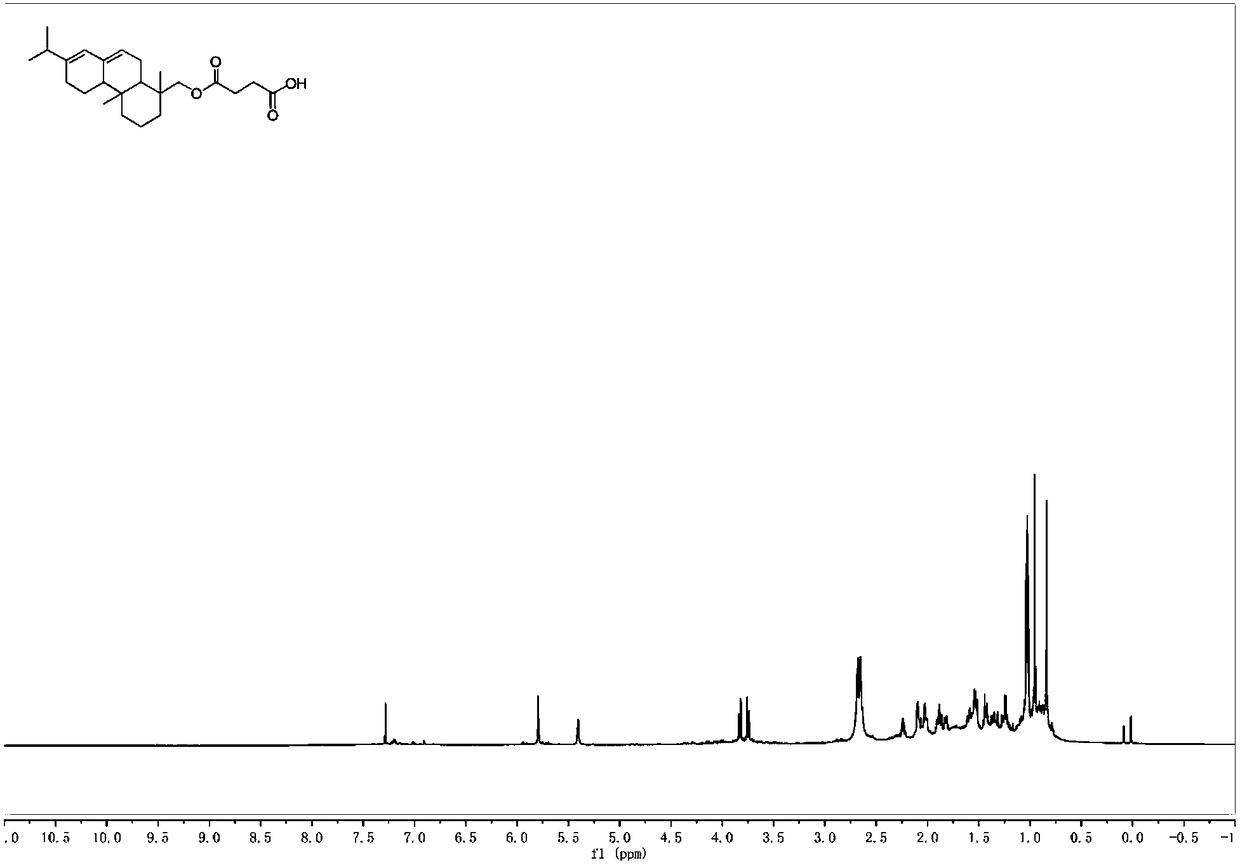
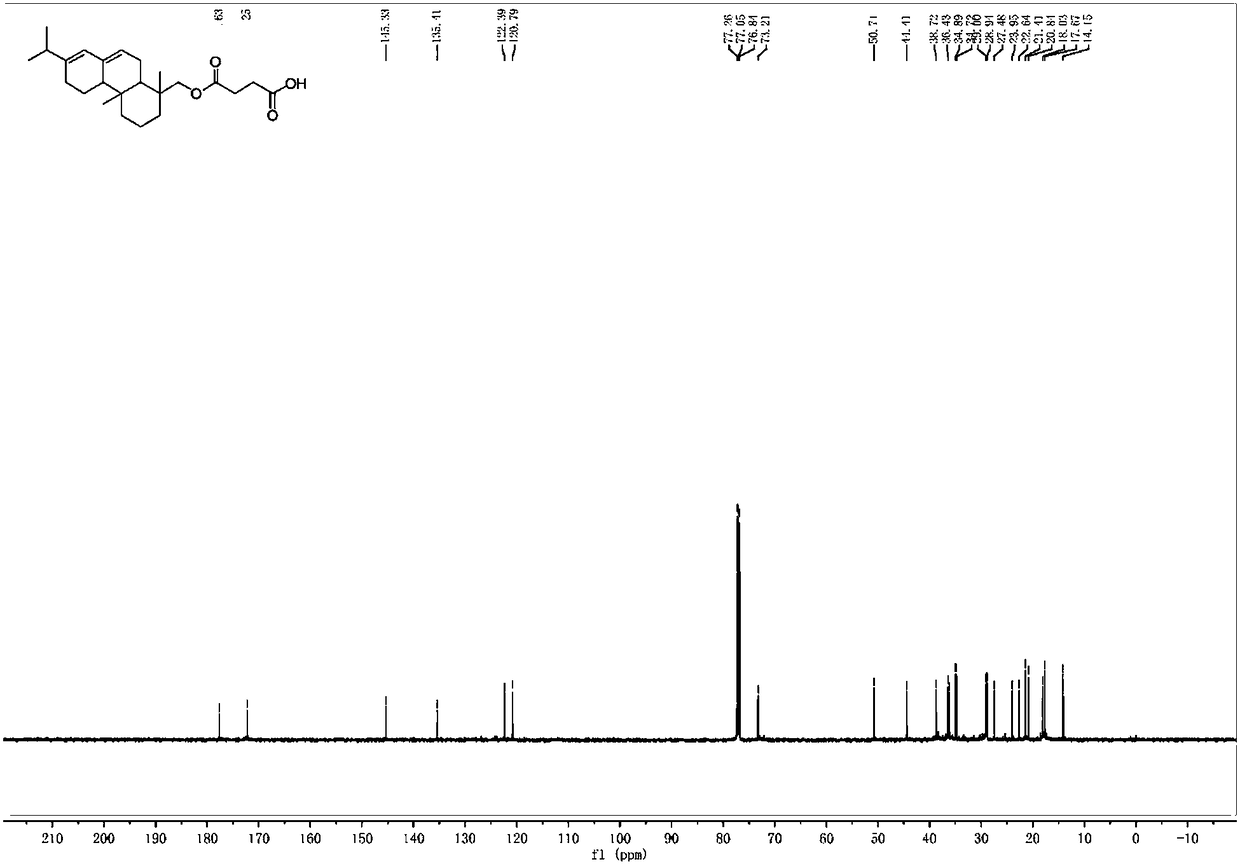
[0040] Add abietyl alcohol (98%, 1.47g, 5mmol) and succinic anhydride (0.8g, 8mmol) into toluene (10mL), mix the system uniformly, add triethylamine (0.175mL, 1.25mmol), heat to 60°C, and 400~700rpm heat preservation and stirring for 5 hours. After the reaction was completed, it was extracted with dichloromethane. After the extracts were combined, they were washed with 2M hydrochloric acid (3×30mL), water (3×30mL) and saturated brine (50mL). The combined organic phases were dried with anhydrous sodium sulfate, rotary evaporation, and column chromatography to obtain abietyl alcohol succinate (1.55 g, 80%). Purity is 98% through HPLC analysis, also can reflect that product purity is extremely high from aspects such as nuclear magnetic spectrum profile, signal, noise).
[0041] 1 H NMR (600MHz, CDCl 3 )δ5.79(s,1H),5.40(s,1H),3.83(d,J=10.9Hz,1H),3.75(d,J=10.9Hz,1H),2.74–2.53(m,7H), 2.26–0.84(m,24H).
[0042] 13 C NMR (151MHz, CDCl 3 )δ177.63,172.25,145.33,135.4...
Embodiment 2
[0044]
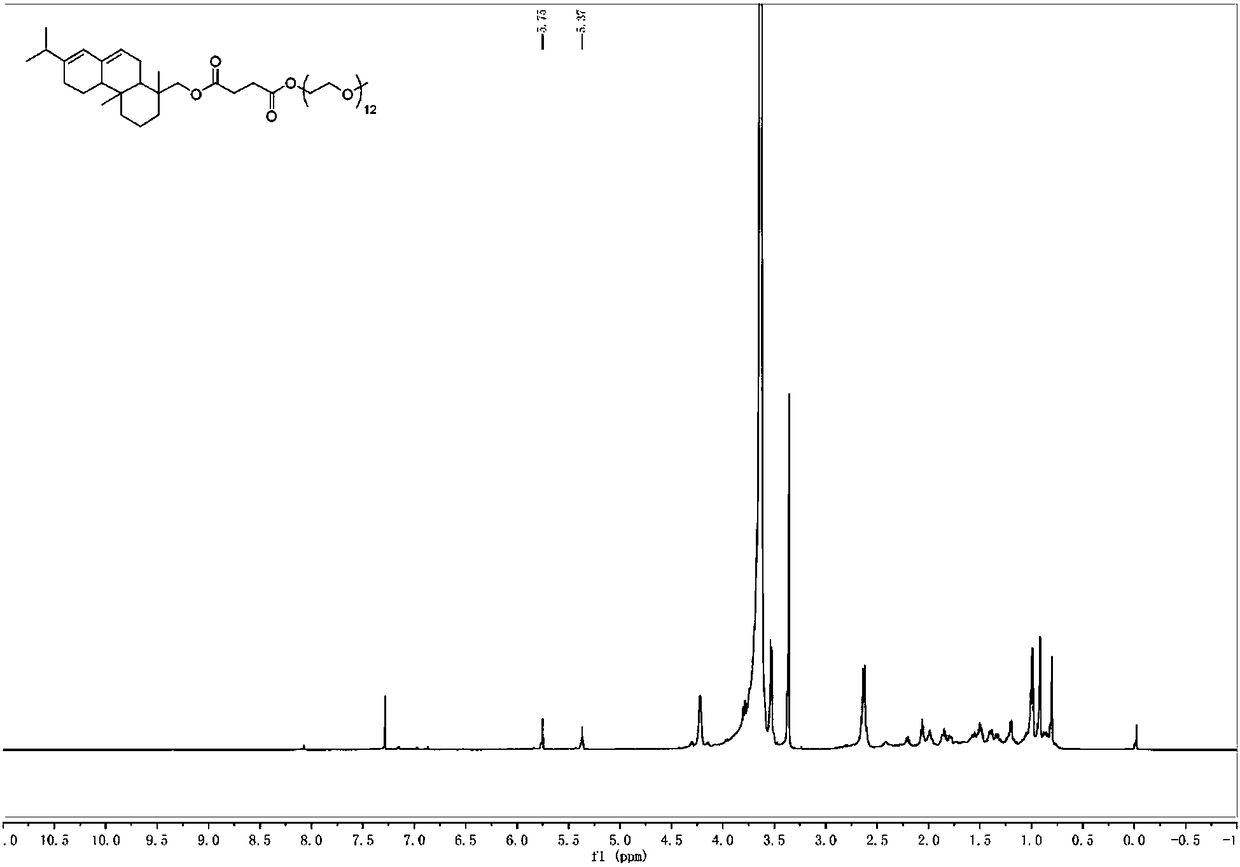
[0045]Arbityl alcohol succinate (98%, 1.31g, 3.39mmol), polyethylene glycol monomethyl ether-550 (2.78g, 5.08mmol), p-toluenesulfonic acid (0.09g, 0.48mmol) were added to toluene (20mL) , heated (130-140° C.) and reacted under reflux for 5 hours. The reactor was equipped with a water belt device, and toluene was used to take out the water generated by the reaction to promote the esterification reaction. After the reaction was completed, saturated sodium bicarbonate was added and extracted with dichloromethane, and the extract was washed with saturated aqueous sodium bicarbonate (3×40 mL) and saturated brine (2×50 mL). The combined organic phases were dried over anhydrous sodium sulfate, rotary evaporated, and column chromatography (developing solvent: ethyl acetate:ethanol=80:20) to obtain the target product 2 (2.65g, 85%). Purity is 98% through HPLC analysis, also can reflect that product purity is extremely high from aspects such as nuclear magnetic spectrum prof...
Embodiment 3
[0049]
[0050] Add dehydroabietyl alcohol (98%, 1.46g, 5mmol), succinic anhydride (0.8g, 8mmol) into toluene (10mL), mix the system uniformly, add triethylamine (0.175mL, 1.25mmol), and heat to 60°C , kept stirring at 400-700rpm for 5 hours. After the reaction was completed, it was extracted with dichloromethane. After the extracts were combined, they were washed with 2M hydrochloric acid (3×30mL), water (3×30mL) and saturated brine (50mL). The combined organic phases were dried over anhydrous sodium sulfate, rotary evaporated, and column chromatographed to obtain dehydroabietyl succinate (1.54 g, 80%). Purity is 98% through HPLC analysis, also can reflect that product purity is extremely high from aspects such as nuclear magnetic spectrum profile, signal, noise).
[0051] 1 H NMR (600MHz, CDCl 3 )δ7.18(d, J=8.2Hz, 1H), 7.00(dd, J=8.1, 1.5Hz, 1H), 6.89(s, 1H), 4.00(d, J=10.9Hz, 1H), 3.76( d,J=10.9Hz,1H),2.86–2.77(m,2H),2.66(dd,J=6.7,5.3Hz,2H),2.61(dd,J=10.0,4.2Hz,2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com