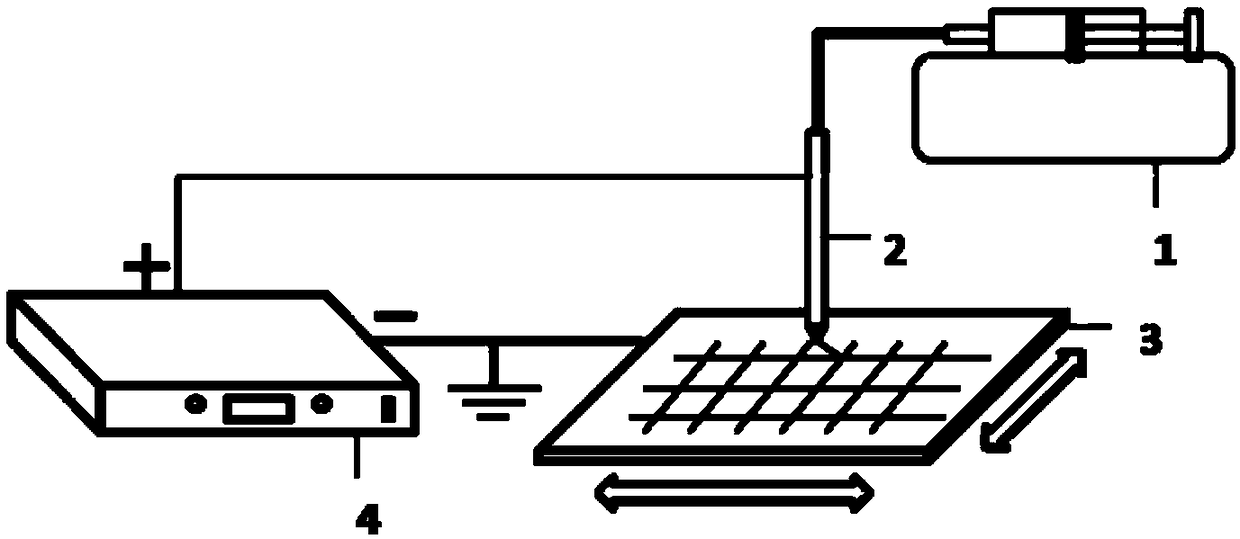
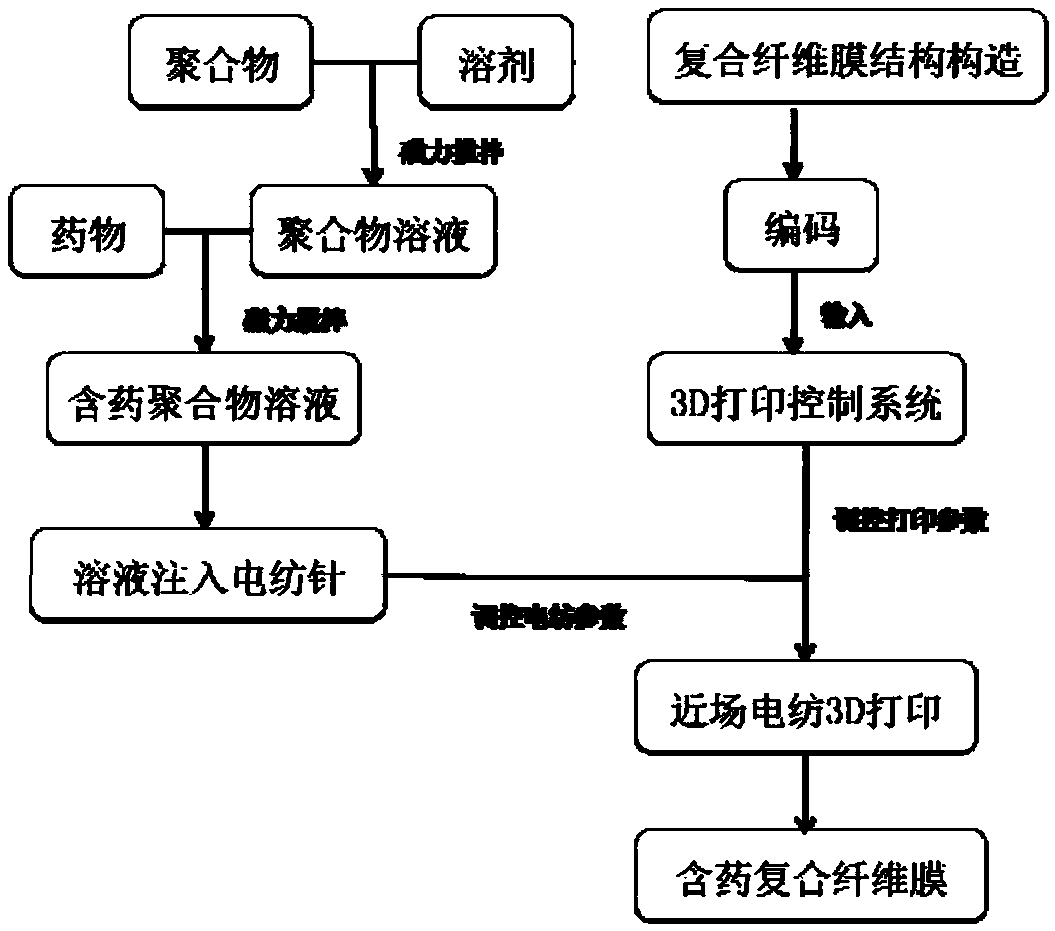
Flexible composite sustained release oral capsule
A capsule and sustained-release technology, applied in the field of drug carrier preparation, can solve the problems of affecting the therapeutic effect, inconvenience in taking, wrong taking, etc., and achieve the effects of strong controllability, wide application range and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
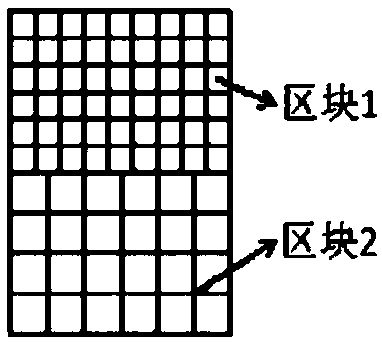
[0045] Write the printing program according to Figure 3(a). The composite fiber membrane is composed of block 1 and block 2 with different grid sizes. ×300μm grid composition. The solute accounts for 40wt% of the electrospinning solution, the solute is polyacrylic acid resin, the solvent is mixed with ethanol and dimethylformamide at a volume ratio of 1:1, and is configured into a polymer solution by magnetic stirring, and the drug concentration is set to 2 %, according to the set concentration, dissolve aspirin into the above polyacrylic acid resin solution, and finally obtain the drug-containing polymer solution as the printing material of block 1; the solute accounts for 22wt% of the electrospinning solution, the solute is cellulose acetate, and the solvent is made of acetone It is mixed with dimethylacetamide at a volume ratio of 1:1, configured into a polymer solution by magnetic stirring, and the drug concentration is set to 1.1%, and pravastatin is dissolved into the abov...
Embodiment 2
[0047] Write the printing program according to Figure 3(b). The composite fiber membrane is composed of block 1 and block 2 with different grid spacing. ×450μm grid composition. The solute accounts for 12% of the electrospinning solution. The solute is composed of polyvinylpyrrolidone and polyoxyethylene in a mass ratio of 1:1, and the solvent is composed of ethanol and water in a volume ratio of 4:1. Through magnetic stirring, the configuration Form a polymer solution, set the drug concentration to 3%, dissolve tetracycline into the above-mentioned zein solution according to the set concentration, and finally obtain a drug-containing polymer solution as the printing material of block 1; the solute accounts for 9wt% of the electrospinning solution, The solute is formed by mixing polylactic acid and polyethylene glycol at a mass ratio of 7:3, and the solvent is formed by mixing dichloromethane and acetone at a volume ratio of 1:1, and is configured into a polymer solution by ma...
Embodiment 3
[0049] Write the printing program according to Figure 3(c). The composite fiber membrane is composed of block 1, block 2 and block 3 with different grid spacings. Block 1 is composed of grids with a size of 80 μm×80 μm. Block 2 Consisting of a grid of size 240 μm × 240 μm, block 3 consists of a grid of size 500 μm × 500 μm. The solute accounts for 18wt% of the electrospinning solution, and the solute is ethyl cellulose, and the solvent is mixed with tetrahydrofuran and dimethylacetamide at a volume ratio of 2:3, and is configured into a polymer solution by magnetic stirring, and the drug concentration is set to 0.5%, according to the set concentration, dissolve captopril into the above-mentioned ethyl cellulose solution, and finally obtain the drug-containing polymer solution as the printing material of block 1; the solute accounts for 26wt% of the electrospinning solution, and the solute is polyhexyl The lactone, the solvent is acetic acid, is configured into a polymer soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com