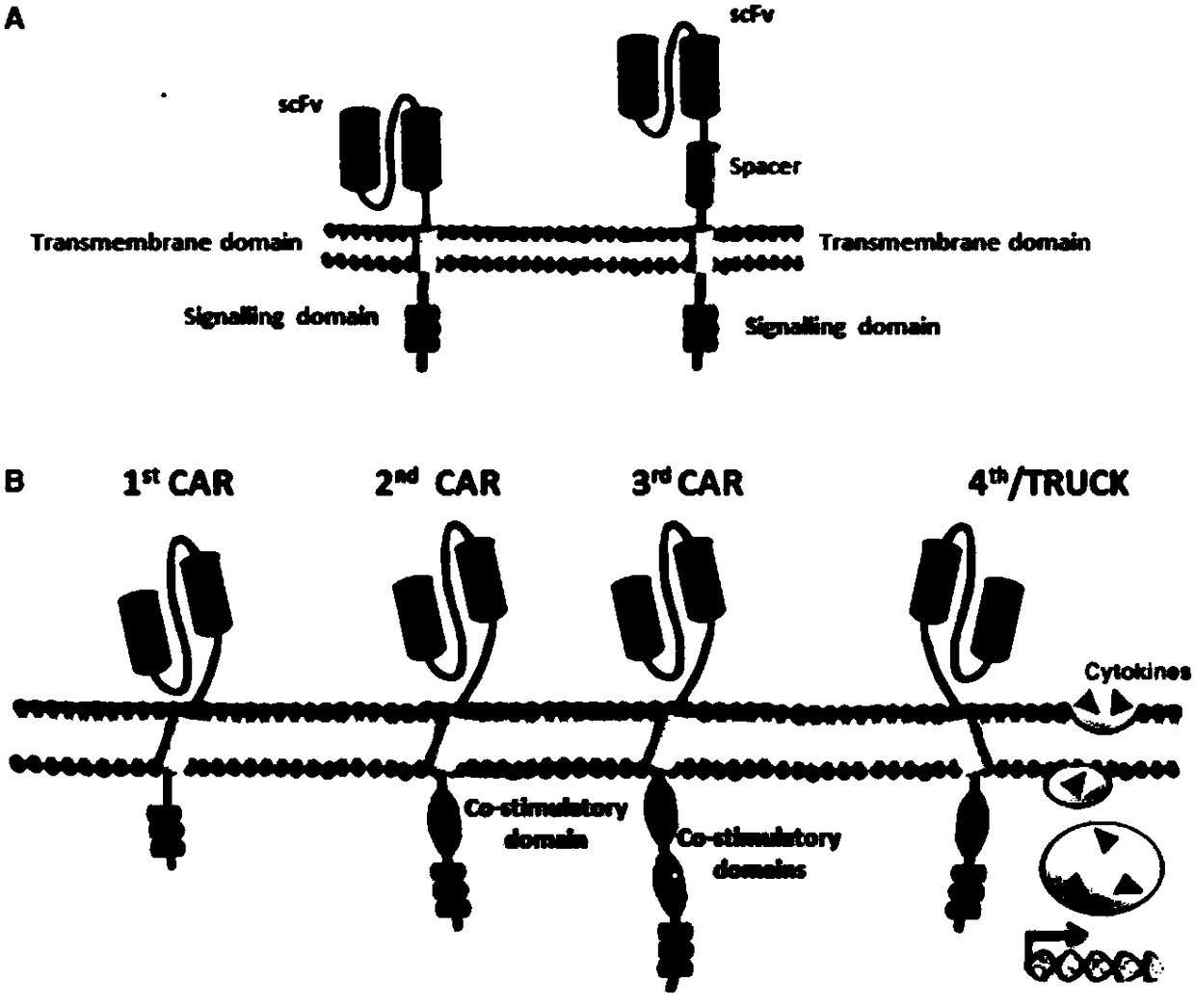
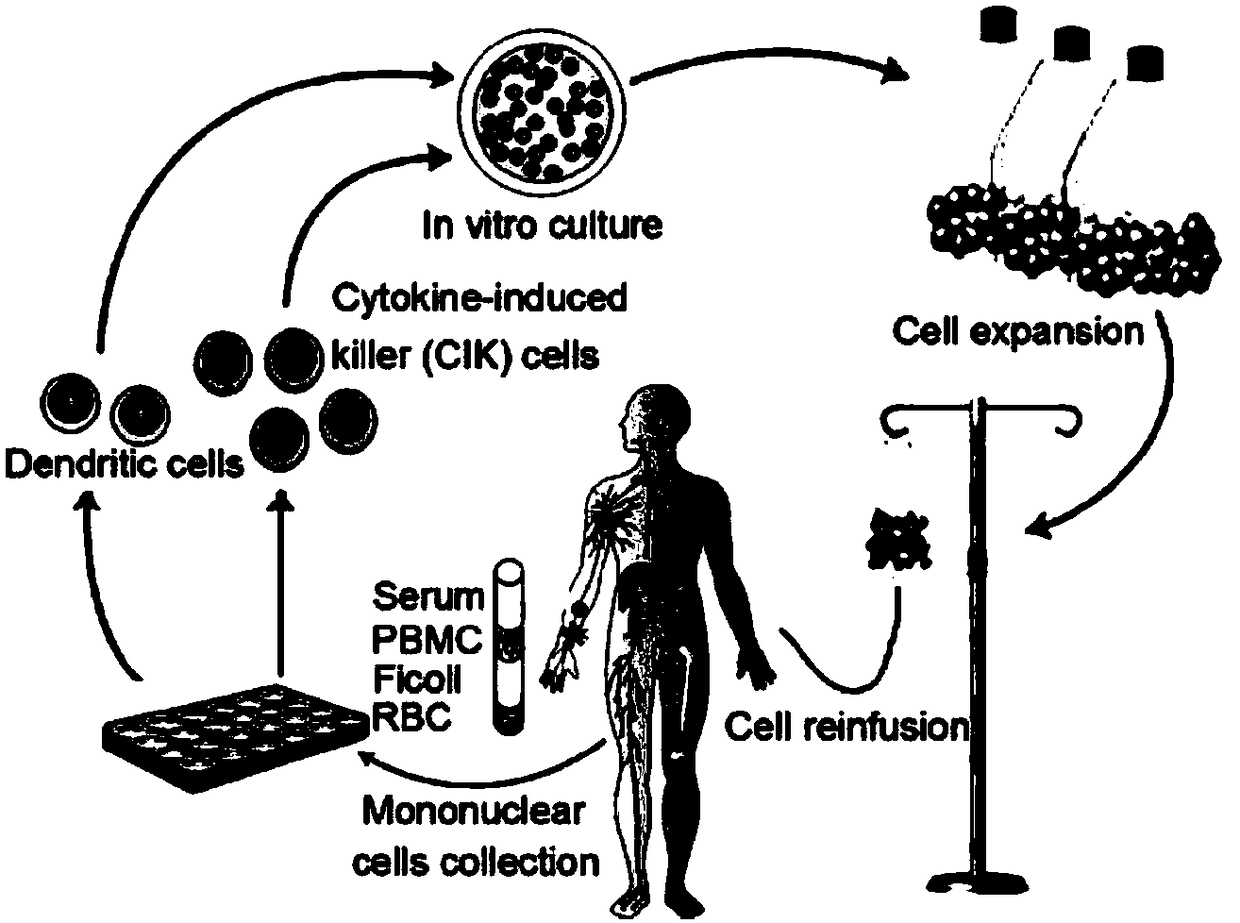
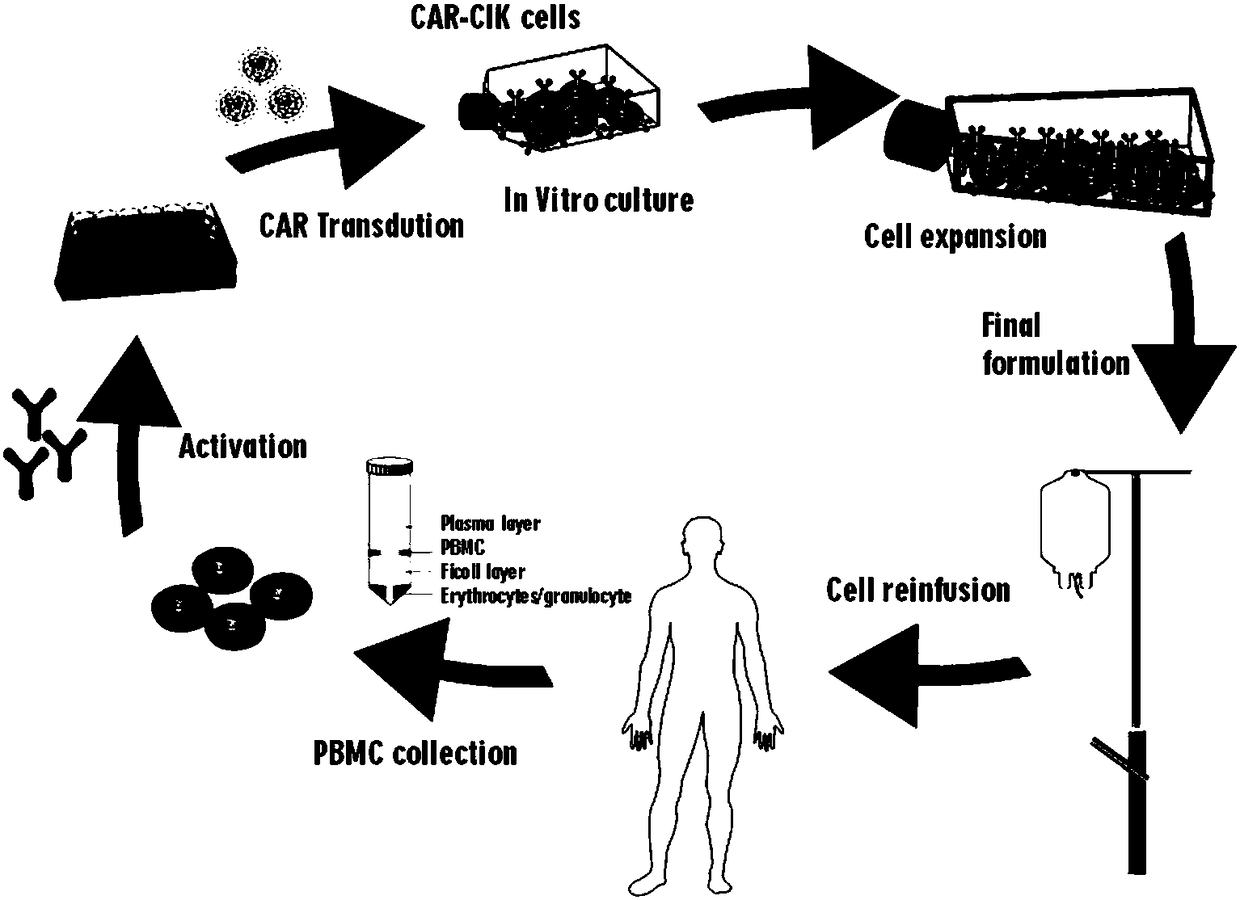
CAR-CIK transgenic cell and preparation method and application thereof
A technology of transgenic cells and transgenic carriers, which is applied in the field of medical biology, can solve the problems of lack of antigen recognition specificity and limitations, and achieve the effects of shortening the culture cycle, increasing survival time, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Construction of CAR-CIK cells.
[0077] see image 3 , the construction method of the CAR-CIK cell described in the present invention is as follows:
[0078] 1. Isolation of PBMCs.
[0079] (1) Take 50ml of fresh peripheral blood from a healthy donor;
[0080] (2) Spray the blood collection bag with alcohol twice and dry it.
[0081] (3) Use a 50ml syringe to suck out the blood cells in the bag and transfer them to a new 50ml tube.
[0082] (4) Centrifuge at 400g for 10 minutes at 20°C.
[0083] (5) Transfer the upper layer of plasma to a new 50ml centrifuge tube, inactivate the plasma at 56°C for 30 minutes, return to room temperature, centrifuge at 2000g for 30 minutes, and take the supernatant into a 50ml centrifuge tube for later use.
[0084] (6) Make up to 50ml with D-PBS(-), tighten the cap, and mix evenly by inversion.
[0085] (7) Take two new 50ml centrifuge tubes and add 15ml Ficoll lymphocyte separation medium to each tube.
[0086] (8) Care...
Embodiment 2
[0115] Example 2 CAR-T cell construction.
[0116] see Figure 4 , the construction method of the CAR-T cell described in the present invention is as follows:
[0117] 1. Isolation of PBMCs.
[0118] (1) Take 50ml of fresh peripheral blood from a healthy donor;
[0119] (2) Spray the blood collection bag with alcohol twice and dry it.
[0120] (3) Use a 50ml syringe to suck out the blood cells in the bag and transfer them to a new 50ml tube.
[0121] (4) Centrifuge at 400g for 10 minutes at 20°C.
[0122](5) Transfer the upper layer of plasma to a new 50ml centrifuge tube, inactivate the plasma at 56°C for 30 minutes, return to room temperature, centrifuge at 2000g for 30 minutes, and take the supernatant into a 50ml centrifuge tube for later use.
[0123] (6) Make up to 50ml with D-PBS(-), tighten the cap, and mix evenly by inversion.
[0124] (7) Take two new 50ml centrifuge tubes and add 15ml Ficoll lymphocyte separation medium to each tube.
[0125] (8) Carefully ad...
Embodiment 3
[0164] Example 3 Pathogen detection and expression detection of CAR-CIK cells and CAR-T cells.
[0165] 1. Endotoxin detection;
[0166] (1), endotoxin working standard is 15EU / branch;
[0167] (2), Limulus reagent sensitivity λ=0.25EU / ml, 0.5ml / tube
[0168] (3) Dilution of endotoxin standard substance: Take one endotoxin standard substance, dilute it with BET water in proportion to dissolve into 4λ and 2λ respectively, seal with parafilm, shake and dissolve for 15min; each step of dilution should be mixed in the vortex Mix on the mixer for 30s;
[0169] (4) Adding samples: Take several LAL reagents, add 0.5 ml of BET water to each tube to dissolve, and distribute to several endotoxin-free test tubes, each tube has 0.1 ml. Two of them are negative control tubes, add 0.1ml of BET water;
[0170] Two are positive control tubes, add 0.1ml of endotoxin working standard solution with 2λ concentration;
[0171] 2 tubes are sample positive control tubes, add 0.1ml sample soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com