Lithium-sulfur battery composite cathode material, preparation method thereof and application of lithium-sulfur battery
A composite positive electrode material and lithium-sulfur battery technology, which is applied in the field of electrochemical energy, can solve the problems of poor cycle performance, low capacity, and high production cost, and achieve high rate performance, high lithium ion conductivity, and stable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Put 1.2g of washed lotus leaf stalks, 1.2g of nickel acetate, and 5g of potassium hydroxide into a mixed solution of 100mL of deionized water and 10mL of alcohol and stir evenly at a speed of 500r / min. Then put it in a vacuum drying oven to dry at 60°C for 5h, and finally put it in a tube furnace, raise the temperature to 850°C at a rate of 5°C / min in an argon atmosphere and keep it for 4h. After the reaction is completed, cool down to room temperature. The solution and deionized water were repeatedly washed to neutrality, and a nickel-doped conductive graphitized carbon material was obtained after freeze-drying at -120°C for 24 hours.
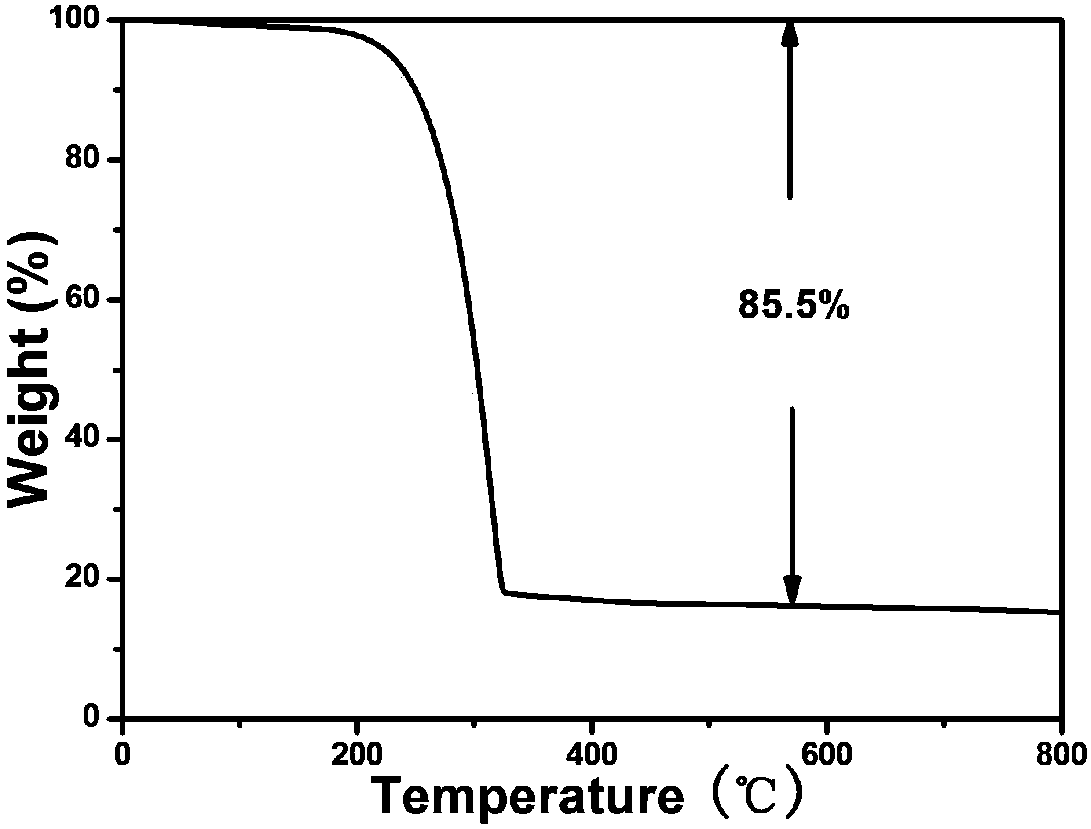
[0051] 0.2g nickel-doped conductive graphitized carbon material was put into CS dissolved with 0.8g elemental sulfur 2 After the organic solution is stirred and mixed, use a syringe to drop the mixed solution on the 2025 battery case or put it into a porcelain boat, heat at 50°C to evaporate all the solvent, and finally place it at a te...
Embodiment 2
[0055] Put 1.2g of washed lotus leaf stalks, 1.74g of nickel nitrate, 0.06g of urea, and 3.58g of potassium hydroxide into a mixed solution of 100mL of deionized water and 10mL of alcohol and stir evenly at a speed of 500r / min. Then put it in a vacuum drying oven for 5 hours at 60°C, and finally put it in a tube furnace, raise the temperature to 500°C at a rate of 1°C / min in an argon atmosphere and keep it for 1 hour. The solution and deionized water were repeatedly washed until neutral, and then freeze-dried at -120°C for 24 hours to obtain a nickel-nitrogen co-doped conductive graphitized carbon material.
[0056] 0.18g nickel-nitrogen co-doped conductive graphitized carbon material and 0.02g PEO (molecular weight 4,000,000) were put into CS dissolved with 0.8g elemental sulfur 2 After the organic solution is stirred and mixed, use a syringe to drop the mixed solution on the 2025 battery case or put it into a porcelain boat, heat at 50°C to evaporate all the solvent, and fin...
Embodiment 3
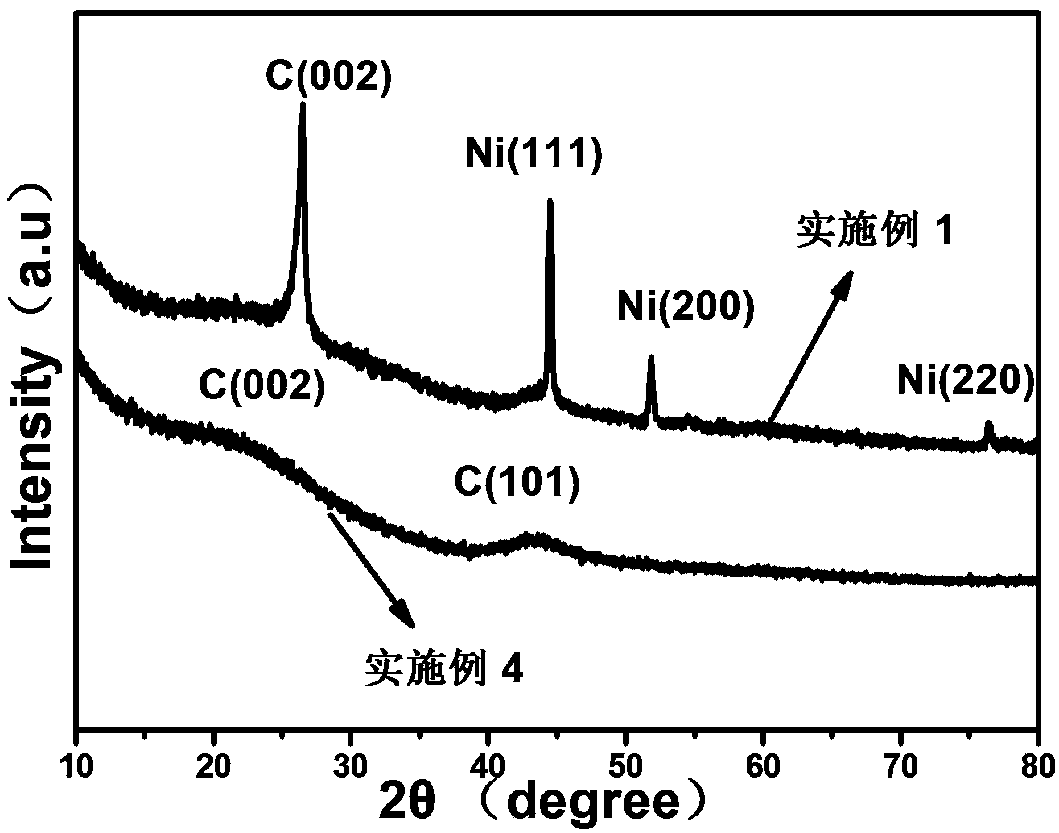
[0060] Put 1.2g of washed cotton stalks, 18.3g of cobalt acetate, 4.2g of sodium fluoride, and 16.8g of sodium hydroxide into a mixed solution of 200mL of deionized water and 50mL of alcohol and stir evenly at a speed of 1000r / min. Then put it in a vacuum drying oven to dry at 60°C for 5h, and finally place it in a tube furnace, raise the temperature to 1000°C at a rate of 10°C / min under an argon atmosphere and keep it for 12h. The solution and deionized water were repeatedly washed to neutrality, and a cobalt-fluorine co-doped conductive graphitized carbon material was obtained after freeze-drying at -120°C for 36 hours. figure 1 As shown in the XRD pattern, the carbon material without metal precursor is amorphous carbon.
[0061] 0.4g cobalt-fluorine co-doped conductive graphitized carbon material and 0.2g PEO (molecular weight of 2000) were put into CS dissolved with 0.4g elemental sulfur 2 After the organic solution is stirred and mixed, use a syringe to drop the mixed so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com