Cell gel preparation for treating articular cartilage injury and use thereof, and used gel solution for maintaining activity of cryopreserved cells
A technology of cell activity and gel solution, which is applied in the fields of biotechnology and biomedicine, can solve the problems of increasing the risk of cell contamination, and achieve the effects of good biocompatibility, high survival rate, and easy preparation and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the gel preparation containing mesenchymal stem cells and / or chondrocytes, the specific steps are as follows:
[0039] ① Preparation of gel solution:
[0040] According to the ratio provided above, the injection-grade or pharmaceutical-grade raw materials, glycerol, human serum albumin, DMSO, chondroitin sulfate, dissolved in compound electrolyte injection and / or amino acid injection, and normal saline injection are replenished, After fully stirring and mixing, set aside.
[0041] ②Acquired cells:
[0042] Select the required cells from the cell bank, use DMEM medium containing 10% fetal bovine serum as the complete medium, and store the cells at 37°C, 5% CO 2When the cells grow to about 85%, the cells are washed with phosphate buffer, digested with 0.25% trypsin, and then the digestion is stopped with complete medium, and the obtained cell suspension is subjected to 1500 × g for 5 min. Centrifuge, discard the supernatant, then resuspend the...
Embodiment 1
[0045] Example 1: Viability detection of umbilical cord mesenchymal stem cells in sodium hyaluronate / collagen / chondroitin sulfate (1:1:0.1) gel
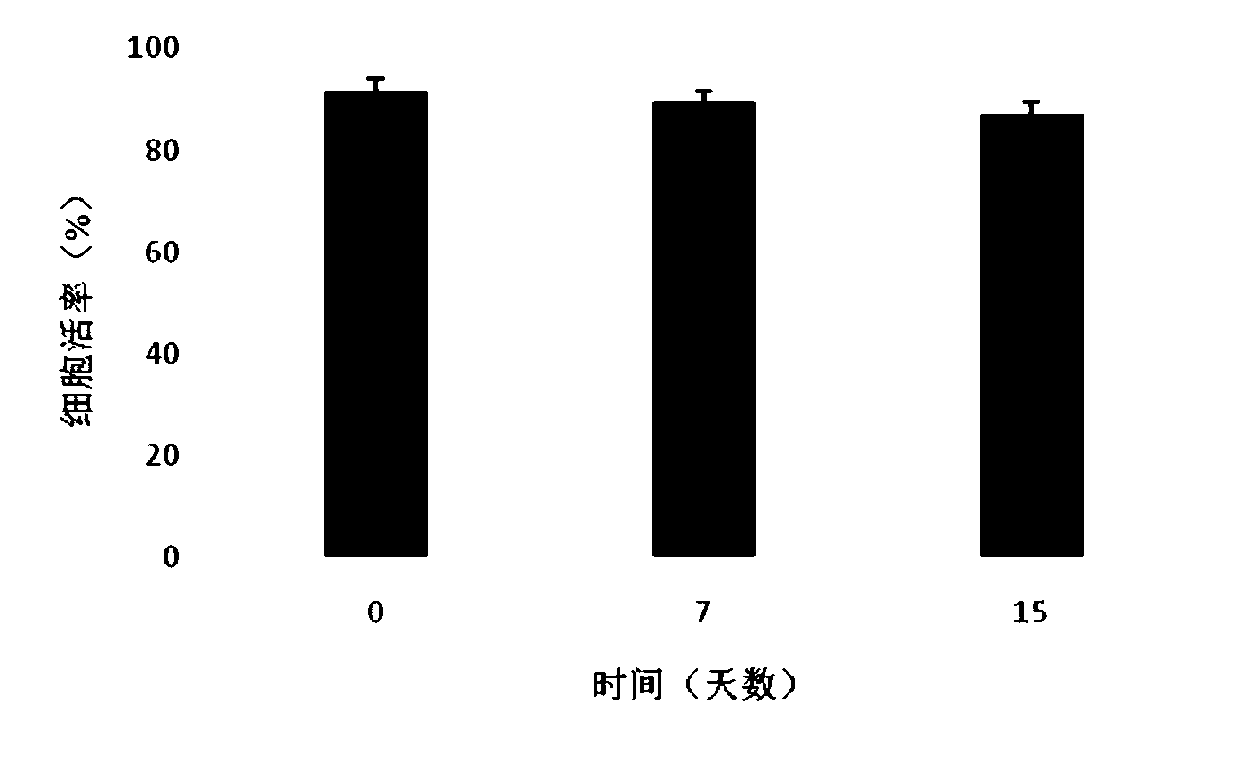
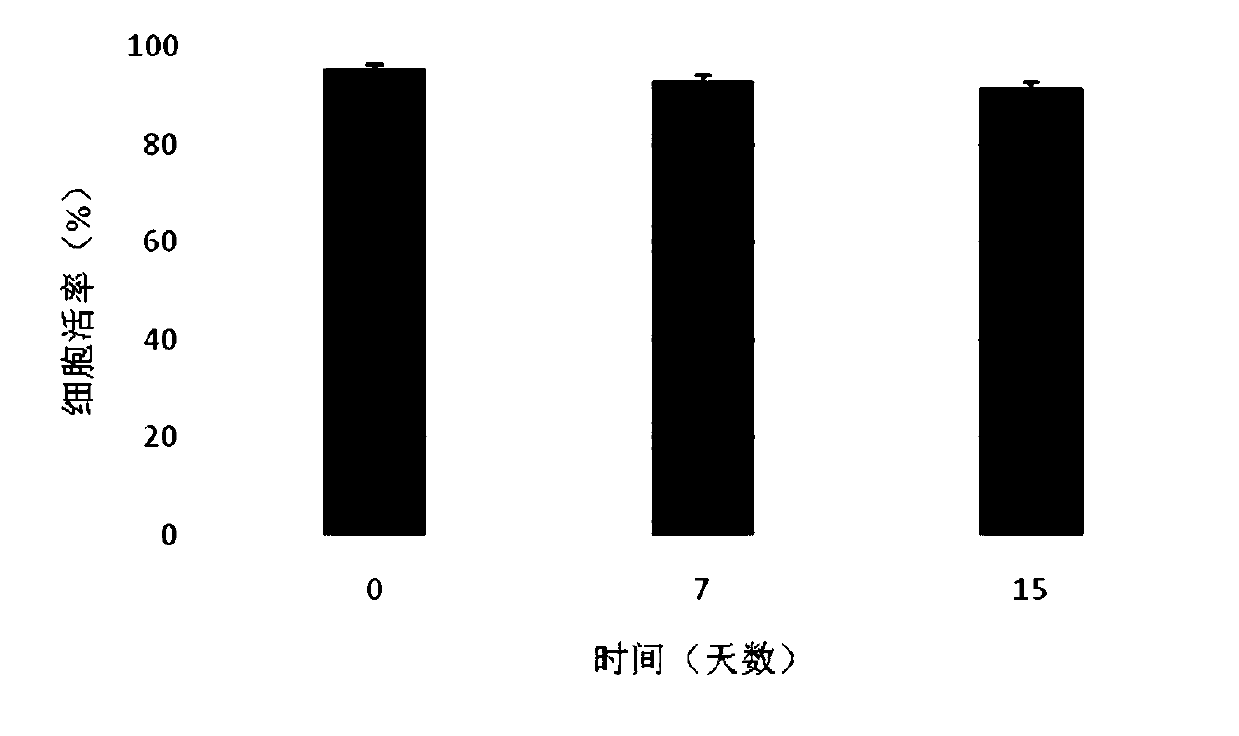
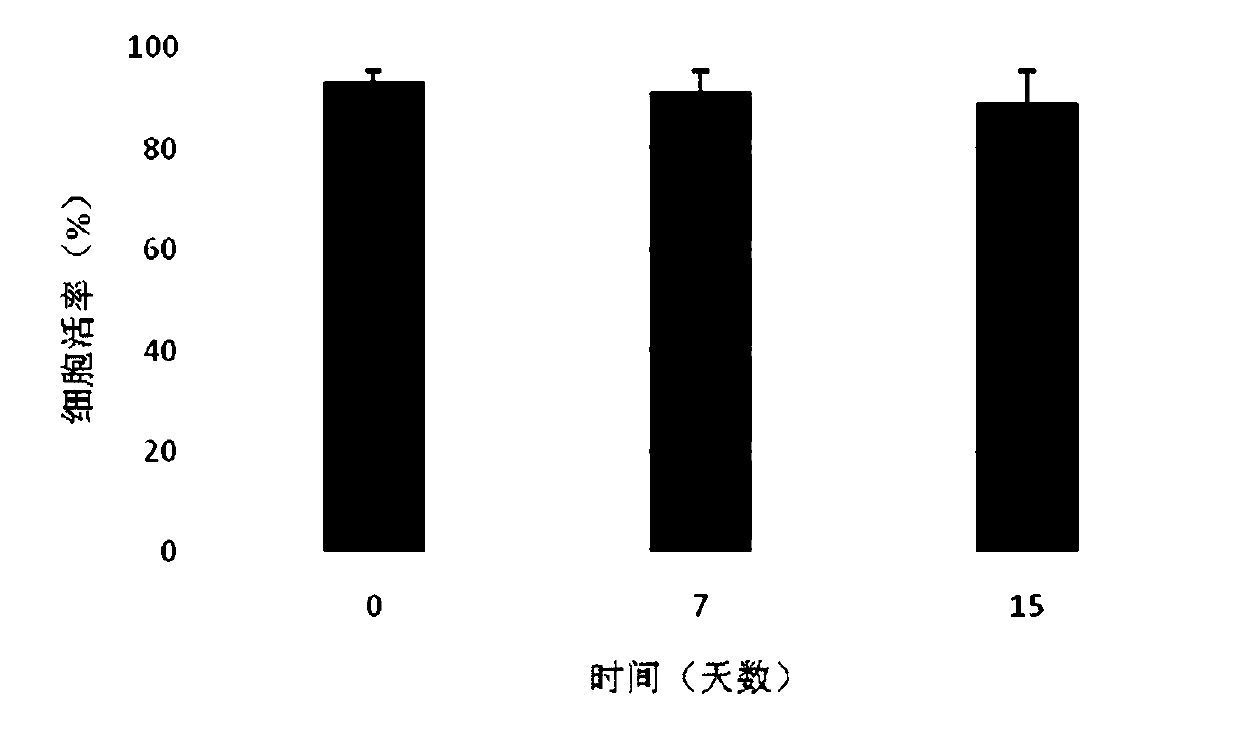
[0046] Combine sterile sodium hyaluronate (10mg), collagen (10mg) and chondroitin sulfate (1mg) into a blended powder, dissolve it in 8.49ml of phosphate solution, swell at room temperature for 24 hours, then add 1.5ml of glycerin , 50mg of human serum albumin, 10ul DMSO, fully stirred and mixed to prepare a polymer material gel solution. After standing still for use, take 900ul gel solution and add 100ul containing 5×10 5 / cells The compound electrolyte resuspension of umbilical cord mesenchymal stem cells, mix well, and make sodium hyaluronate / chondroitin sulfate / collagen gel preparation. They were directly frozen and stored in a -80°C refrigerator. After 0, 7, and 15 days, 3 tubes were taken from each group for cell viability detection. It can be seen from the experimental results that the gel preparation has good cytocompatibil...
Embodiment 2
[0047] Example 2: Viability detection of umbilical cord mesenchymal stem cells in sodium hyaluronate / collagen / chondroitin sulfate gel (48:2:1)
[0048] Combine sterile sodium hyaluronate (480mg), collagen (20mg) and chondroitin sulfate (10mg) into blended powder, dissolve in 9.4ml amino acid injection, swell at room temperature for 24 hours, then add 300ul glycerol, 5mg of human serum albumin, 300ul DMSO, fully stirred and mixed, prepared polymer material gel solution. After standing still for use, take 900ul gel solution and add 100ul containing 5×10 5 / cells The compound electrolyte resuspension of umbilical cord mesenchymal stem cells, mix well, and make sodium hyaluronate / collagen / chondroitin sulfate gel preparation. They were directly frozen and stored in a -80°C refrigerator. After 0, 7, and 15 days, 3 tubes were taken from each group for cell viability detection. It can be seen from the experimental results that the gel preparation has good cytocompatibility, that is,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com