N,N-diethyl-2-(2-methoxy-5-nitrophenoxyl)-ethylamine as well as preparation method and application thereof
A technology of nitro and chlorotriethylamine hydrochloride, which is applied in the preparation of amino hydroxyl compounds, botany equipment and methods, applications, etc., and can solve the problems of rare new varieties of plant growth regulators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
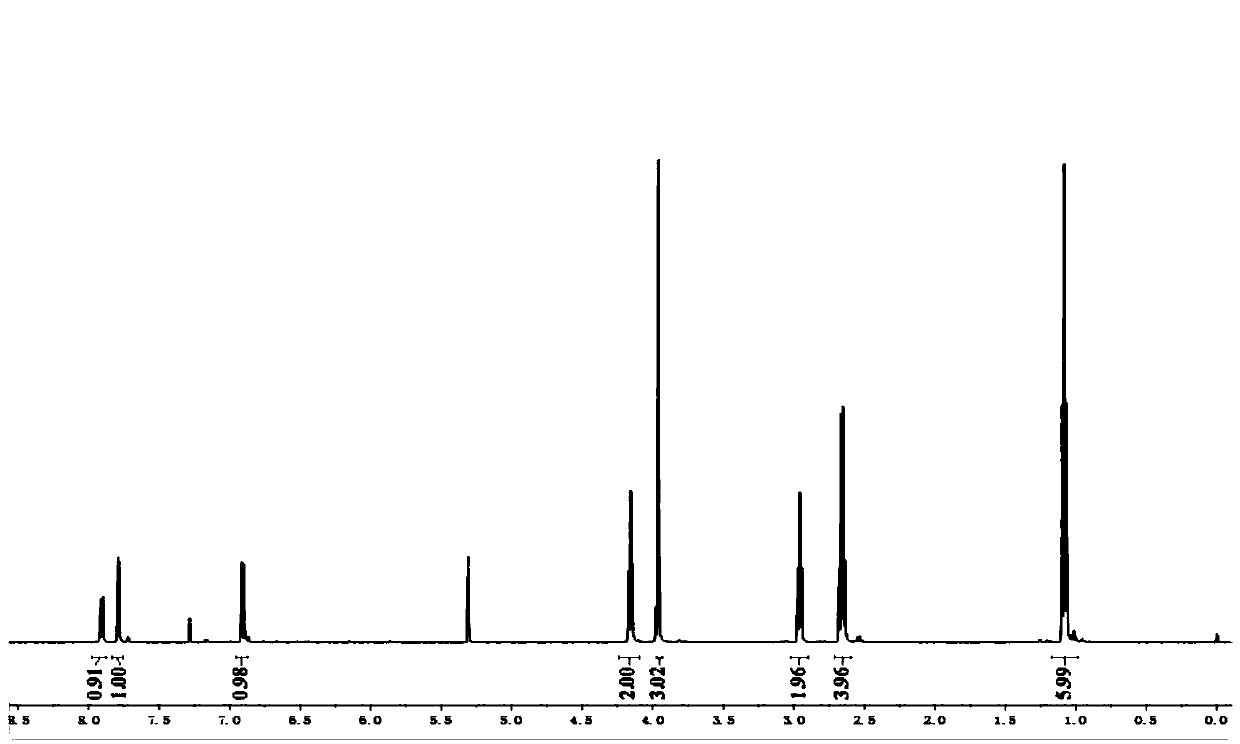
Image
Examples
preparation example Construction
[0040] The present invention also provides a preparation method for the compound described in the above technical scheme, comprising the following steps:
[0041] 1) dissolving 5-nitroguaiacol sodium and tetrabutylammonium bromide in aqueous sodium hydroxide solution to obtain a mixed solution;
[0042] 2) Dissolving β-chlorotriethylamine hydrochloride in water to obtain an aqueous solution of β-chlorotriethylamine hydrochloride;
[0043] 3) heating the mixed solution to 80° C., adding dropwise the β-chlorotriethylamine hydrochloride aqueous solution to the heated mixed solution to carry out a nucleophilic substitution reaction to obtain the compound shown in formula I;
[0044] There is no time sequence limitation between the steps 1) and 2).
[0045] The invention uses 5-nitroguaiacolate sodium as a raw material, and dissolves the 5-nitroguaiacolate sodium and tetrabutylammonium bromide in an aqueous sodium hydroxide solution to obtain a mixed solution. The present inventi...
Embodiment 1
[0071] In a 250ml four-necked flask, add 9g sodium 5-nitroguaiacol, 1.5g tetrabutylammonium bromide, and 100g mass concentration of 10% sodium hydroxide aqueous solution successively, stir and dissolve, add 50g water after dissolving, Raise the temperature to 800°C to obtain a reaction system;
[0072] 10g of β-chlorotriethylamine hydrochloride was dissolved in 36g of water, filtered to remove impurities, and then poured into a constant pressure dropping funnel;
[0073] The β-chlorotriethylamine hydrochloride solution is slowly added dropwise to the above reaction system, and the dropping time is controlled at about two hours. The progress of the reaction was followed by thin layer chromatography until the reaction was completed.
[0074] The obtained reaction solution was poured into a 250ml separatory funnel for layering while hot, and the organic phase was separated;
[0075] Cool the remaining aqueous phase to room temperature, wash with dichloromethane, and combine the...
Embodiment 2
[0080] In a 250ml four-necked flask, add 9g sodium 5-nitroguaiacol, 1g tetrabutylammonium bromide, and 100g aqueous sodium hydroxide solution with a mass concentration of 10% in sequence, stir and dissolve, add 50g water after dissolving, and heat up to 80°C to obtain a reaction system;
[0081] 9g of β-chlorotriethylamine hydrochloride was dissolved in 40g of water, filtered to remove impurities, and then poured into a constant pressure dropping funnel;
[0082] The β-chlorotriethylamine hydrochloride solution is slowly added dropwise to the above reaction system, and the dropping time is controlled at about two hours. The progress of the reaction was followed by thin layer chromatography until the reaction was completed.
[0083] The obtained reaction solution was poured into a 250ml separatory funnel for layering while hot, and the organic phase was separated;
[0084] Cool the remaining aqueous phase to room temperature, wash with dichloromethane, and combine the organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com