Cesium-lead halide perovskite crystal materials with high photoelectric response efficiency and stability at room temperature as well as preparation method and application of cesium-lead halide perovskite crystal materials
A photoelectric response and crystal material technology, applied in polycrystalline material growth, chemical instruments and methods, crystal growth, etc., can solve the problems of high-concentration ion doping difficulties and large differences in solubility, etc., to reduce dark current density, The effect of widening the absorption range and increasing the photocurrent density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take 10.276g PbBr 2 Place in a 50mL beaker and stir to dissolve completely, then add 2.982g of CsBr, raise the temperature to 120°C, and stir until completely dissolved. The beaker was sealed with plastic wrap, and a 1 cm long thin line-shaped opening was drawn. Put the solution in an oil bath at 120 °C, the solvent evaporates slowly, and CsPbBr is precipitated when it reaches supersaturation 3 crystals. The crystals were taken out, washed quickly with DMF, and the solution attached to the surface of the crystals was removed after repeated washings.
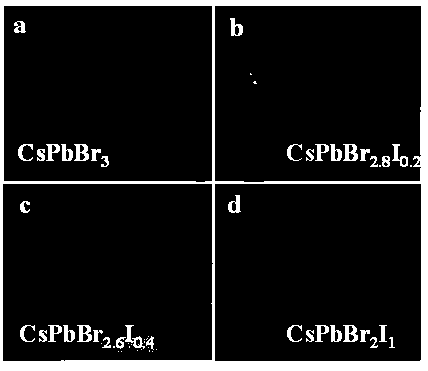
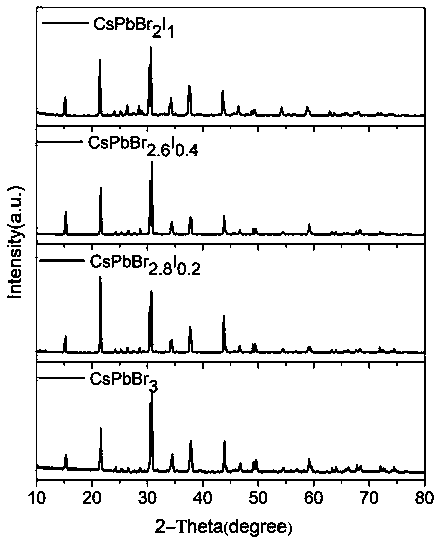
[0036] Photographs of the obtained crystals are shown in figure 2 Shown in a. figure 2 Middle a shows CsPbBr 3 The crystal is transparent, the crystal quality is high, and the size is large, its size is 5.10×4.91×1.43mm 3 . The crystalline phase of the prepared product was analyzed by Japan Rigaku Mini Flex II type X-ray powder diffraction test, the results are as follows image 3 . by CsPbBr 3 The X-ray powder...
Embodiment 2
[0038] Take 9.248g PbBr 2 Place in a 50mL beaker and stir to dissolve completely, then add 1.290g PbI 2 Stir to make it dissolve completely, finally add 2.982g of CsBr, raise the temperature to 120°C, and stir until completely dissolved. The beaker is sealed with a plastic wrap, and a 1 cm long thin line-shaped opening is drawn. Put the solution in an oil bath at 120°C, the solvent evaporates slowly, and CsPbBr is precipitated when it reaches supersaturation 2.8 I 0.2 crystals. The crystals were taken out, washed quickly with DMF, and the solution attached to the surface of the crystals was removed after repeated washings.
[0039] Photographs of the obtained crystals are shown in figure 2 Shown in b. figure 2 Middle b shows CsPbBr 2.8 I 0.2 The crystal is transparent, the crystal quality is high, and the size is large, its size is 5.60×4.60×1.56mm 3 . The crystalline phase of the prepared product was analyzed by Japan Rigaku Mini Flex II type X-ray powder diffract...
Embodiment 3
[0042] Take 8.236g PbBr 2 Place in a 50 mL beaker and stir to dissolve completely, then add 2.5816g PbI 2 Stir to dissolve completely, then add 2.982g CsBr, raise the temperature to 120°C, and stir until completely dissolved. The beaker is sealed with plastic wrap, and a 1 cm long thin line-shaped opening is drawn. Put the solution in an oil bath at 120°C, the solvent evaporates slowly, and CsPbBr is precipitated when it reaches supersaturation 2.6 I0.4 crystals. The crystals were taken out, washed quickly with dimethyl sulfoxide, and the solution attached to the surface of the crystals was removed after repeated washings.
[0043] Photographs of the obtained crystals are shown in figure 2 As shown in c. figure 2 Middle c shows CsPbBr 2.6 I 0.4 The crystal is relatively transparent, the crystal quality is high, and the size is large, and its size is 3.70×3.40×2.00mm 3 . The crystalline phase of the prepared product was analyzed by Japan Rigaku Mini Flex II type X-ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com