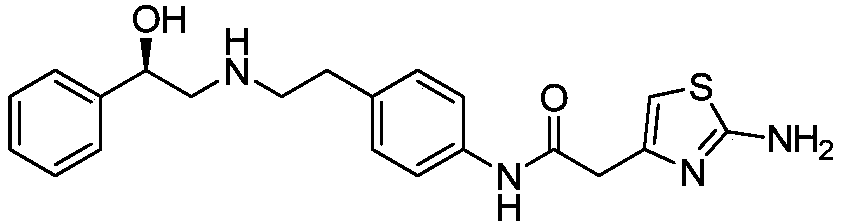
Synthesis of mirabegron intermediate (R)-2-hydroxy-N-(4-nitrophenyl ethyl)-2-phenylacetamide
A technology of nitrophenethyl and nitrophenethylamine, which is applied in the field of synthesis of Mirabegron intermediate -2-hydroxy-N--2-phenylacetamide, can solve the problem of high price condensation by-products, The post-processing steps are cumbersome, the product purity is not high, etc., and the industrial operation is feasible, the post-processing is simple, and the yield is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
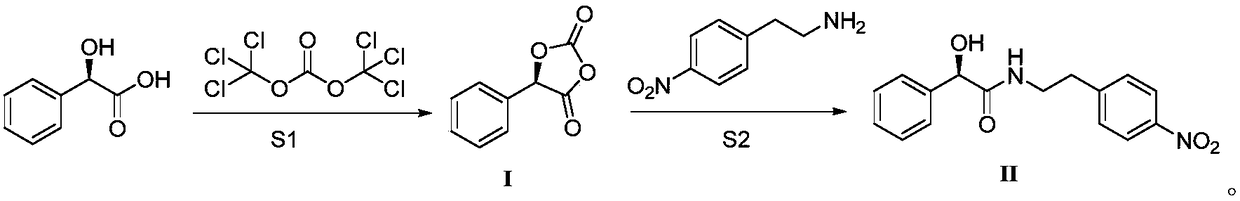
[0027] Preparation of Intermediate I: Under mechanical stirring, add 15.2g R-mandelic acid, 22.2g triethylamine, 32.5g triphosgene and 150mL acetonitrile into a 250mL three-neck flask, react at 25°C for 6h, complete the reaction in TLC, and cool to room temperature , filtered, the solvent was concentrated in vacuo, 150mL ethyl acetate and 150mL water were added to the residue, stirred for half an hour, the layers were left to stand, the organic phase was dried, and concentrated to give 17.7g of a white solid, namely intermediate I, with a yield of 99%. 1 H NMR (400MHz, CDCl 3 )d 6.02(s, 1H), 7.40-7.46(m, 5H).
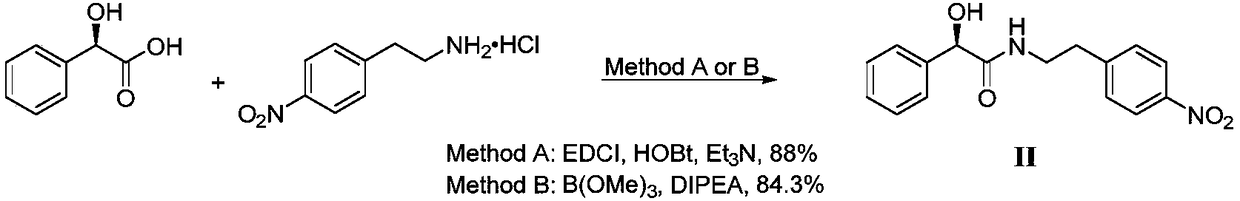
[0028] Preparation of the target product: Dissolve 8.9g of intermediate I in 90mL of acetone, add 9.1g of 4-nitrophenylethylamine, react at 56°C for 3 hours, the reaction in TLC is completed, and the solvent is concentrated in vacuo at room temperature, and 30mL of Dissolve methanol at 50°C, cool down and crystallize for 12 hours, filter, wash with 15mL of methanol, an...
Embodiment 2
[0030] Preparation of Intermediate I: Add 30g of R-mandelic acid, 60g of potassium carbonate, 65g of triphosgene and 300mL of DMF into a 500mL three-neck flask under mechanical stirring, and react at 30°C for 5h. After the reaction is completed in TLC, cool to room temperature, filter, and vacuum Concentrate the solvent, add 300mL ethyl acetate and 300mL water to the residue, stir for half an hour, let stand to separate layers, dry the organic phase, and concentrate to obtain 35.5g of white solid, Intermediate I, with a yield of 99%.
[0031] Preparation of the target product: Dissolve 18g of intermediate I in 150mL THF, add 18.2g of 4-nitrophenylethylamine, react at 40°C for 3 hours, the reaction is completed in TLC, and the solvent is concentrated in vacuo at room temperature, and 60mL of methanol is added Dissolve at 50°C, cool down and crystallize for 12 hours, filter, wash with 30mL of methanol, and dry to obtain 13.7g of white solid, namely Mirabegron intermediate (R)-2-h...
Embodiment 3
[0033] Preparation of Intermediate I: Add 30g of R-mandelic acid, 26g of DMAP, 65g of triphosgene and 300mL of acetonitrile into a 500mL three-neck flask under mechanical stirring, react at 15°C for 8h, the reaction is controlled by TLC, cool to room temperature, filter, and concentrate in vacuo Solvent, add 300mL ethyl acetate and 300mL water to the residue, stir for half an hour, let stand to separate layers, dry the organic phase, and concentrate to obtain 35.5g of white solid, namely intermediate I, with a yield of 99%.
[0034] Preparation of the target product: Dissolve 18g of intermediate I in 150mL of acetonitrile, add 18.2g of 4-nitrophenylethylamine, react at 70°C for 3 hours, the reaction is completed in TLC, and the solvent is concentrated in vacuo at room temperature, and 60mL of methanol is added Dissolve at 50°C, cool down and crystallize for 12 hours, filter, wash with 30mL of methanol, and dry to obtain 13.4g of white solid, which is the Labegron intermediate (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com