Organic compound for detecting histamine and application of organic compound
A technology of organic compounds and compounds, applied in the field of fluorescent probes, to achieve the effect of reducing interference and improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
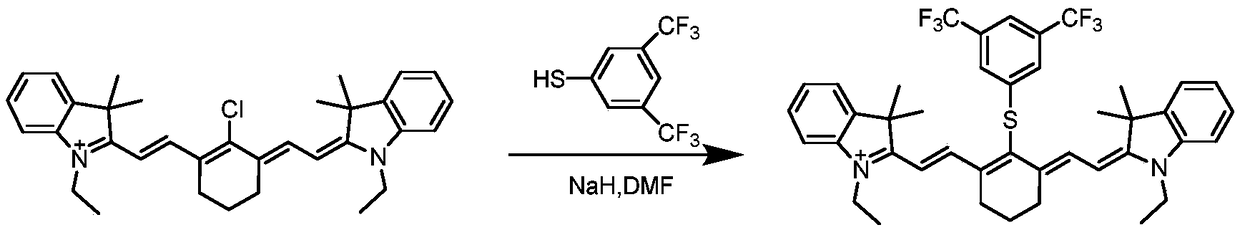
[0032] Synthesis of the compound shown in formula I: under ice-bath conditions, 3,5-bistrifluoromethylthiophenol (264mg, 1.07mmol) was dissolved in 80ml of anhydrous DMF and placed in a 250ml round-bottomed flask. 20ml of anhydrous DMF dissolved NaH (430mg, 1.07mmol) and placed in a constant pressure dropping funnel and dripped into the reaction bottle, stirred for 20min, and then commercialized cyanine dye Cy 7 -Cl (640mg, 1mmol) was added to the reaction system, such as figure 2 . Reacted for 90 minutes, extracted three times with dichloromethane and NaI aqueous solution, the organic layer was rotary evaporated to remove the solvent, and the crude product was purified by column chromatography to obtain 704 mg of the green product of the compound represented by formula I, with a yield of 83%.
[0033] 1 H NMR (500MHz, CDCl 3 )δ(ppm): 1 H NMR (500MHz, CDCl 3 -D 1 )δ8.59-8.56(d,2H),7.67(s,2H),7.59(s,1H),7.42–7.36(m,4H),7.27–7.24(m,4H),6.31-6.28(d, 2H), 4.30-4.28(q, 4H),...
Embodiment 2
[0035] Spectral properties of compound formula Ⅰ
[0036] The prepared compound of formula I is used as a probe in water system, simulated physiological environment and cells to detect histamine. The following experiments are all carried out under the condition of pH=7.4 (HEPES buffer solution, concentration is 10mM), the concentration of probe 1 mM was used.
[0037] The compound of formula I prepared above is used as the response of probe to histamine:
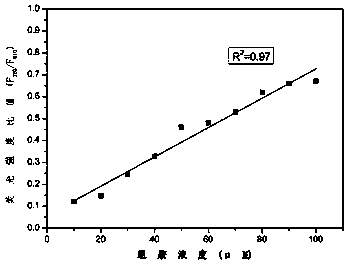
[0038] Add 100 μL of the compound of Formula I (1mM) to a 10mL colorimetric tube, dilute to 10.0mL with 10mM HEPES buffer solution (pH 7.4), shake the solution for 10min and take 1mL in a fluorescent cuvette to measure the fluorescence spectrum at 750nm-860nm . Add 1mL of histamine (100μM) to the above colorimetric tube to make up to 10mL, shake the solution to balance for 10min, absorb 1mL of the working solution in the colorimetric tube into a fluorescent dish to measure the fluorescence spectrum, and measure the fluores...
Embodiment 3
[0040] The selectivity of compound formula I to histamine
[0041] Take nine 10mL colorimetric tubes, and add 100 μL of the compound of formula I to each colorimetric tube, then add 8 mL of HEPES buffer solution with a pH of 7.4, and then add 1 mL of the substance to be tested (100 μM): (1) histamine, (2) Histidine, (3) Tryptophan, (4) Ammonia, (5) Alanine, (6) Glutathione, (7) Cysteine, (8) Hydrogen peroxide, ( 9) Ascorbic acid. Finally, the volume was adjusted to 10ml with HEPES buffer. Shake the solution at room temperature and equilibrate in the dark for 10 minutes, pour 1 mL of the working solution in each colorimetric tube into a fluorescent dish to measure the fluorescence spectrum in the range of 680nm-800nm (see Figure 5 ).
[0042] Depend on Figure 5 From the experimental results, it can be concluded that the compound of formula Ⅰ, as a probe, has a selective response to histamine and is not interfered by other biological amino acids, active sulfur, and activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com