Purification process for sodium fluoride and silicon dioxide
A silicon dioxide and sodium fluoride technology, applied in the direction of silicon dioxide, silicon oxide, alkali metal fluoride, etc., can solve problems affecting product quality and achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
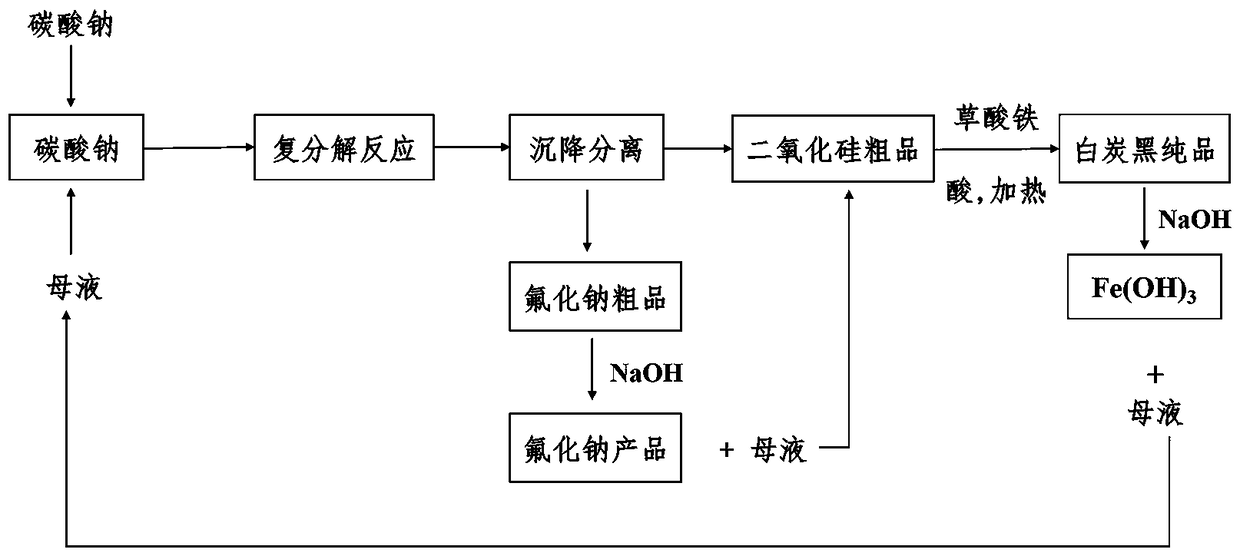
[0024] Example 1: A separation and purification process of sodium fluoride and silica, which uses sodium fluorosilicate, a by-product of phosphate fertilizer, to produce sodium fluoride and co-produce white carbon black. The process includes the following steps:
[0025] (1) Metathesis reaction: Weigh 482 g of solid sodium carbonate, add 1100 g of mother liquor to prepare a 30% solution, heat to 85°C, press Na 2 SiF 6 : Na 2 CO 3 =1:1.05~1.1 for batching, slowly add 400 g of solid sodium fluorosilicate, the feeding time is 30 minutes, and the reaction is continued at 90 ℃ for 1 hour to obtain a solution of sodium fluoride crystals and silica precipitation;
[0026] (2) Preliminary separation: The mixed solution of sodium fluoride and silicon dioxide is settled in a separating scrubber, and then the supernatant is removed to obtain 529.6 g crude sodium fluoride and 123.0 g crude silica. The crude sodium fluoride contains 4.00 g silica, and the crude silica contains 3.76 g sodium fluo...
Embodiment 2
[0029] Example 2: A separation and purification process of sodium fluoride and silicon dioxide, which uses sodium fluorosilicate, a by-product of phosphate fertilizer, to produce sodium fluoride and co-produce white carbon black. The process includes the following steps:
[0030] (1) Metathesis reaction: Weigh 482 g of solid sodium carbonate, add 482 g of mother liquor to prepare a 50% solution, raise the temperature to 95°C, press Na 2 SiF 6 : Na 2 CO 3 =1:1.05~1.1 for batching, slowly add 400 g of solid sodium fluorosilicate, the feeding time is 30 minutes, and the reaction is continued at 90 ℃ for 1 hour to obtain a solution of sodium fluoride crystals and silica precipitation;
[0031] (2) Preliminary separation: The mixed solution of sodium fluoride and silicon dioxide is settled in a separating scrubber, and then the supernatant is removed to obtain 529.6 g crude sodium fluoride and 123.0 g crude silica. The crude sodium fluoride contains 4.00 g of silica, and the crude silica...
Embodiment 3
[0034] Example 3: A separation and purification process of sodium fluoride and silica, which uses sodium fluorosilicate, a by-product of phosphate fertilizer, to produce sodium fluoride and co-produce white carbon black. The process includes the following steps:
[0035] (1) Metathesis reaction: Weigh 482 g of solid sodium carbonate, add 1100 g of mother liquor to prepare a 30% solution, heat to 90°C, press Na 2 SiF 6 : Na 2 CO 3 =1:1.05~1.1 for batching, slowly add 400 g of solid sodium fluorosilicate, the feeding time is 30 minutes, and the reaction is continued at 90 ℃ for 1.5 hours to obtain a solution of sodium fluoride crystals and silica precipitation;
[0036] (2) Preliminary separation: The mixed solution of sodium fluoride and silicon dioxide is settled in a separating scrubber, and then the supernatant is removed to obtain 529.6 g crude sodium fluoride and 123.0 g crude silica. The crude sodium fluoride contains 4.00 g silica, and the crude silica contains 3.76 g sodium f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com